
Recent studies suggest that moderate AS is not a benign condition and is associated with increased mortality and morbidity especially when LV function is already reduced.
For health care professionals
Now enrolling



The objective of the PROGRESS trial is to evaluate the safety and effectiveness of the Edwards SAPIEN 3, SAPIEN 3 Ultra, and SAPIEN 3 Ultra RESILIA transcatheter heart valves compared with clinical surveillance in patients with moderate, calcific aortic stenosis (AS).
Patients will be randomized 1:1 to receive either TAVR with the SAPIEN 3 valve platform (via the transfemoral approach) or clinical surveillance
Contact our Patient Support Center to find a participating hospital near you
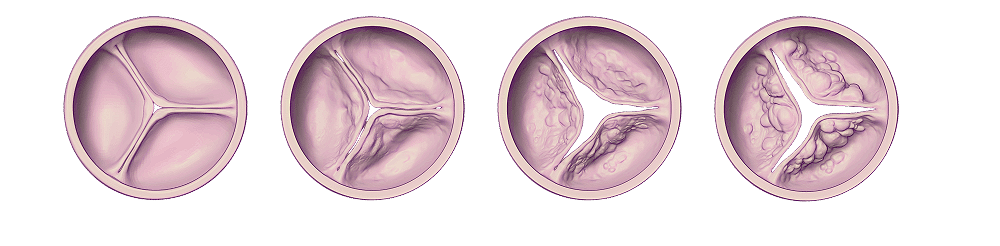
Aortic stenosis is known to be a progressive disease with a highly variable and unpredictable rate of progression. Different degrees of obstruction lead to a spectrum of adaptive and eventually pathological cardiac mechanisms, ranging from left ventricular (LV) hypertrophy, myocardial fibrosis, LV depression, and subsequent consequences extending beyond the aortic valve and LV (i.e., atrial fibrillation, secondary mitral regurgitation, pulmonary artery hypertension, right ventricle dysfunction)

Recent studies suggest that moderate AS is not a benign condition and is associated with increased mortality and morbidity especially when LV function is already reduced.

Histopathology and cardiac magnetic resonance imaging have shown that progressive pressure overload impacts the LV with fibrosis seen even before AS is severe.

Recent data suggest that the rate of progression from mild-moderate AS to more severe AS is highly variable, unpredictable, and that some subgroups of patients with moderate AS are associated with a worse prognosis.

The PROGRESS trial will investigate if earlier intervention may help improve outcomes in patients with moderate AS and clinical characteristics associated with worse prognosis.





We are committed to providing the highest levels of customer service to help our patients improve their quality of life. For any questions, or if you are interested in being part of this study, please contact the Edwards Patient Support Center. For details about the trial, visit NCT04889872 at Clinical Trials.gov.
Give us a call
Send us an email
The Edwards SAPIEN 3 and Edwards SAPIEN 3 Ultra transcatheter heart valves are investigational devices when used in patients with moderate aortic stenosis. Limited by Federal (USA) law to investigational use only. These devices are not available for marketing or commercial sale in the United States for patients with moderate aortic stenosis.