Track patient progress on your smartphone
Seamlessly access your patients' HemoSphere monitor data from anywhere.
Stay connected to continuous patient insights from anywhere
Edwards Lifesciences Critical Care product group is now part of BD (Becton, Dickinson and Company). Edwards is the manufacturer of the products.
To find the latest information, please visit this page on BD.com
With Viewfinder remote app, you can view insights for multiple patients directly from your smartphone. Stay connected in the moment with remote access to hemodynamic* and tissue oximetry insights, trends, alerts and more. Plus, streamline charting with seamless, secure connection to your hospital's EMR system.
Seamlessly access your patients' HemoSphere monitor data from anywhere.
Examine data collected from patients who are connected to HemoSphere monitors, so you’ll always have visibility.

Improve clinical workflow with remote access to continuous insights on your patients’ hemodynamic stability.
*Acumen Assisted Fluid Management (AFM) software feature not included on Viewfinder remote app. Viewfinder remote app is intended as a visual support aid, not as a monitoring device.
HemoSphere platform brings comprehensive insights seamlessly onto one monitor, including predictive parameters for hypotension management, continuous arterial waveform tracing and blood pressure measurements, advanced hemodynamic parameters, tissue oximetry insights and more.
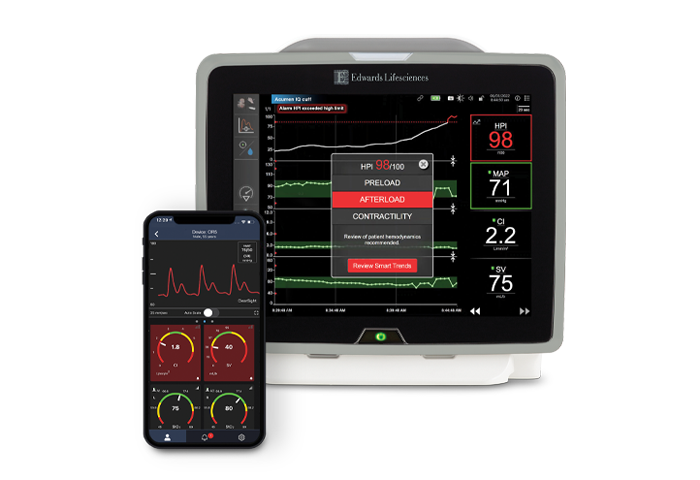
With Viewfinder remote app on your smartphone, you will receive the same insights you would see at your patients’ bedside, all from the palm of your hand: noninvasive arterial waveform, historical trend data, alarms, alerts and more.**
Viewfinder remote app opens up new possibilities. Remote access to continuous noninvasive advanced hemodynamic monitoring and patient alarm notifications helps improve clinical response time, so you can stay ahead of unstable moments.
Viewfinder remote app can be used as an educational tool – creating visibility across the patient care continuum and improving communication across the care team.
**Please refer to the Operator’s Manual for smartphone compatibility. Acumen Assisted Fluid Management (AFM) software feature not included on Viewfinder remote app.
Viewfinder remote app enables you to stay informed of hemodynamic instability with access to HemoSphere monitor’s complete insights.
Simultaneously view insights for any patients connected to HemoSphere monitors. Keep track of your patients with complete, continuous and customizable access to hemodynamic status.
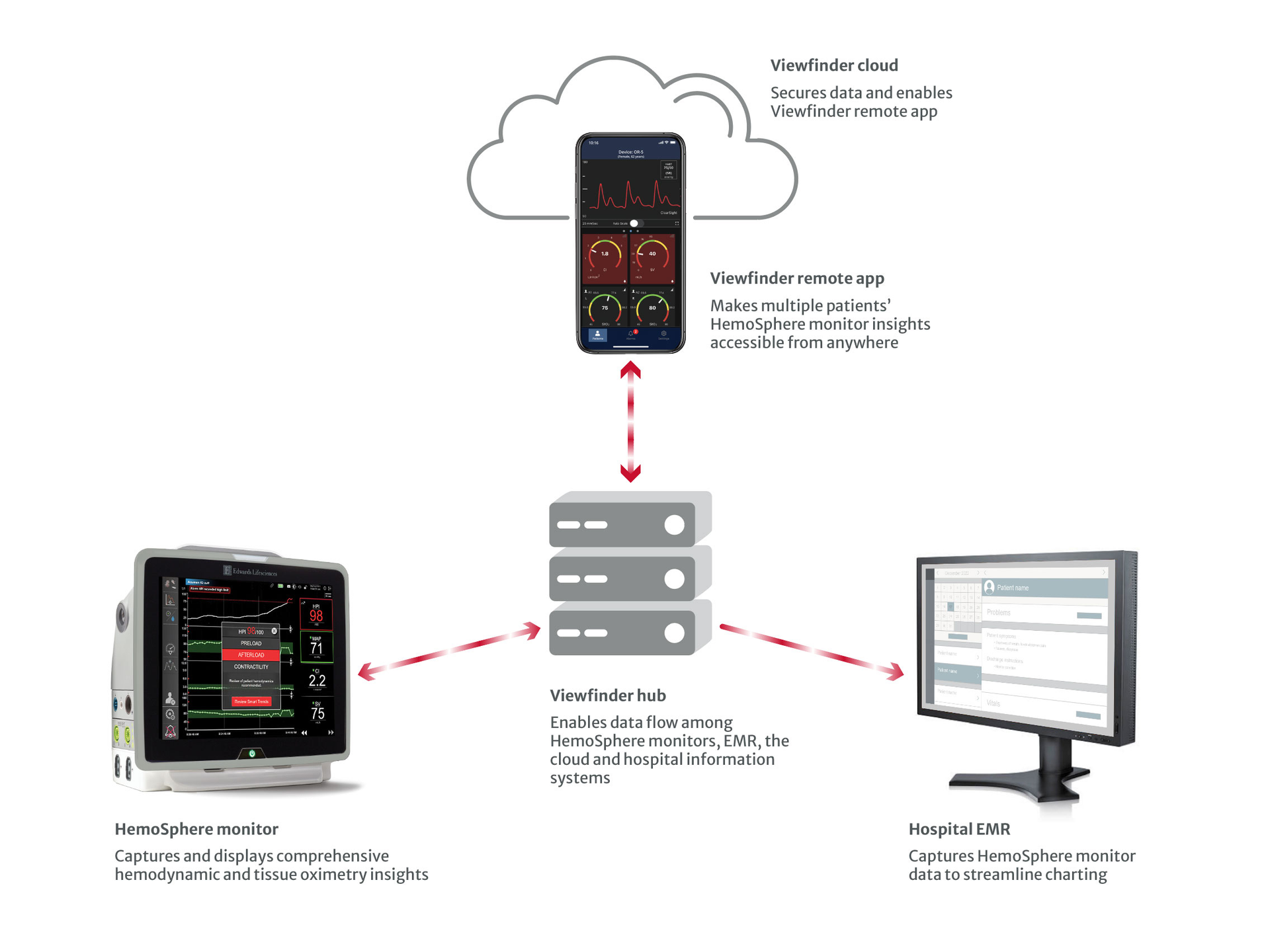
Viewfinder remote app is just one part of a comprehensive and secure data ecosystem that makes Viewfinder network possible. Viewfinder hub, the centralized secure data exchange software, integrates with your hospital information systems and Viewfinder remote app to enable wireless data flow from HemoSphere monitors to your EMR system.
With seamless, secure connectivity, you can spend less time charting and more time with patients.
Viewfinder pathways app, our interactive hemodynamic protocol library, enables you to take key protocols with you wherever you go.
Access key hemodynamic monitoring protocols from anywhere:

Viewfinder pathways app is a protocol library designed to help inform clinicians of key published protocols used to guide hemodynamic monitoring.

Viewfinder pathways app interactive flowcharts guide you through each protocol to assist in training and education – and are downloadable to your phone, tablet or computer.

Create a free account to make Viewfinder pathways app your single source for protocols. Add favorites, filter by procedure type – even upload your hospital’s own protocols to your library.
Discover how Viewfinder remote app works. This instructional video covers adding patient monitoring sessions, remotely following multiple patient sessions at once, accessing different monitoring views and more.
Viewfinder remote app enables full access to your patients’ HemoSphere monitor insights.
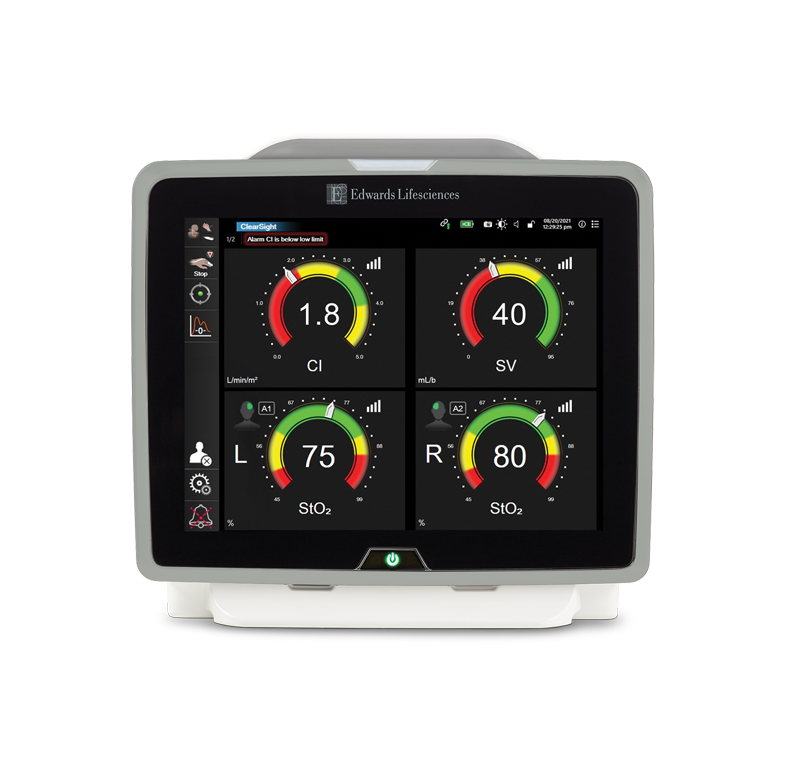
Provides a comprehensive view of hemodynamics and tissue oximetry sensor on one monitor.
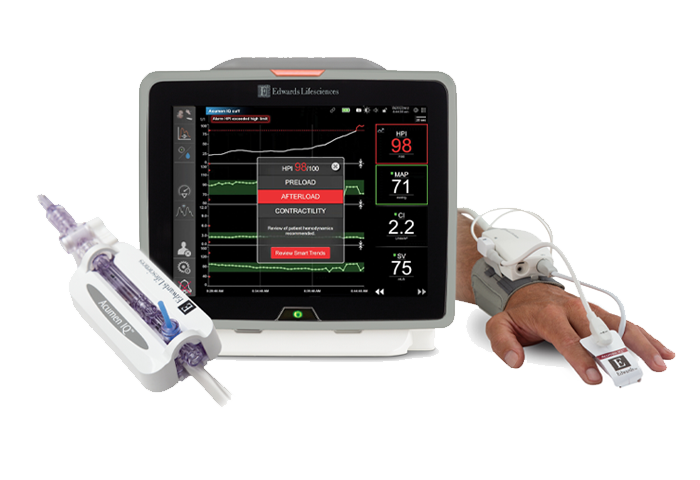
Unlocked by Acumen IQ sensor (minimally invasive) or Acumen IQ cuff (noninvasive), Acumen HPI software is a first-of-its-kind predictive decision support software that can detect the likelihood of a patient trending towards hypotension.

ForeSight sensor delivers absolute StO2 values that - when used in combination with the full hemodynamic insights delivered by HemoSphere monitor - enable you to recognize and reverse cerebral desaturations.
CAUTION: Federal (United States) law restricts this device to sale by or on the order of a physician.
See Instructions For Use (IFU) / Directions For Use (DFU) for full prescribing information, including indications, contraindications, warnings, precautions and adverse events.
We are committed to providing your institution, clinicians and staff with the highest levels of customer service and support to ensure seamless product implementation and ongoing use, including:
24/7 Technical support
For product information and orders