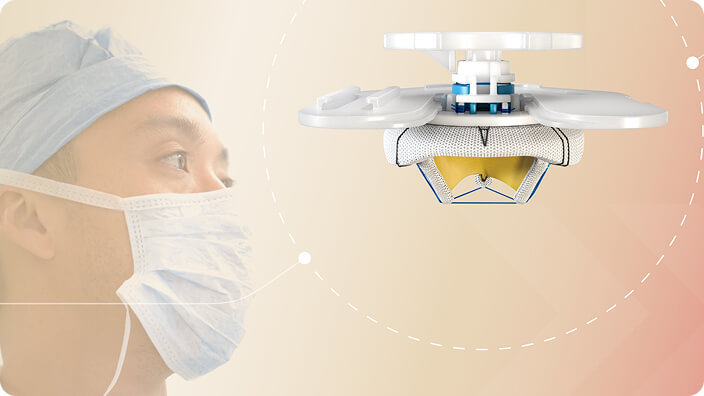
Optimized for ease of use
Learn about how the MITRIS valve was designed to optimize usability
Designed to handle the pressure of the mitral position

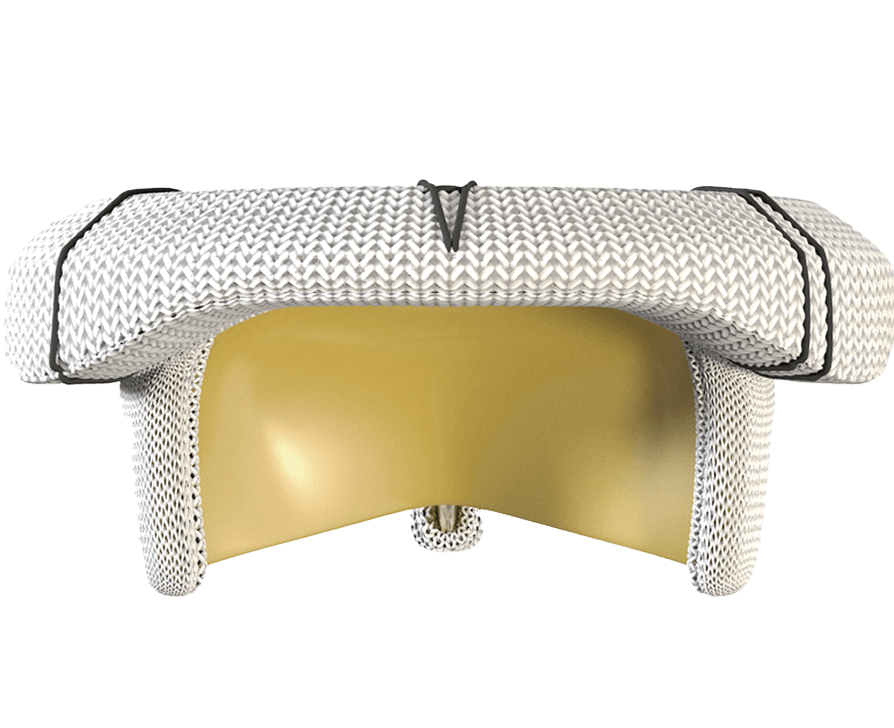
* Clinical data on valves with RESILIA tissue up to 7-year follow-up have been published, with additional follow-up of up to 10 years in process.
‡The safety and effectiveness of ViV in the MITRIS valve has not been established.
Indications: For use in replacement of native or prosthetic mitral heart valves.
Contraindications: There are no known contraindications with the use of the MITRIS RESILIA mitral valve.
Complications and Side Effects: Thromboembolism, valve thrombosis, hemorrhage, hemolysis, regurgitation, endocarditis, structural valve deterioration, nonstructural dysfunction, stenosis, arrhythmia, transient ischemic attack/stroke, congestive heart failure, myocardial infarction, ventricular perforation by stent posts, any of which could lead to reoperation, explantation, permanent disability, and death.
CAUTION: US law restricts this device to sale by or on the order of a physician. See Instructions for Use for full prescribing information, including indications, contraindications, warnings, precautions and adverse events.