RESILIA tissue

RESILIA tissue is building a track record of study data.
Edwards Lifesciences is committed to continued scientific advancement, and studies that investigate the safety and performance of new technologies. Learn more about the current methodologies, promising results and conclusions of the various RESILIA tissue studies below.
Watch Dr. Tsuyoshi Kaneko, Chief of Cardiac Surgery at Washington University in St. Louis, present the latest RESILIA tissue valve data: Propensity-matched 8-year outcomes following surgical aortic valve replacement with novel versus contemporary tissue bioprostheses1
Clinical data on valves with RESILIA tissue up to 7-year follow-up have been published, with additional follow-up to 10-years in progress.
Beaver T, Bavaria JE, Griffith B, et al. Seven-year outcomes following aortic valve replacement with a novel tissue bioprosthesis. J Thorac Cardiovasc Surg. 2024;168(3):781-791. doi:10.1016/j.jtcvs.2023.09.047
RESILIA tissue valves: 8-year results
In a propensity score-matched analysis, RESILIA tissue valves significantly outperformed contemporary tissue bioprostheses in SAVR patients
Methods
Study design
This analysis compares long-term outcomes between RESILIA tissue and non-RESILIA tissue valves using propensity-matched data from two prospective trials.
Baseline characteristics, pre-matching
RESILIA tissue valves*
- Sample size: 689 patients
- Mean age: 66.9 years
- Study design: Prospective, multicenter, single-arm
- Events adjudication: Clinical events committee
- SVD definition: Akins C, et al. Ann Thorac Surg. 20082.
Non-RESILIA tissue valves‡
- Sample size: 258 patients
- Mean age: 68.5 years
- Study design: Prospective, multicenter, single-arm
- Events adjudication: Clinical events committee
- Akins C, et al. Ann Thorac Surg. 20082.
For a detailed breakdown of the study design, methodology, and results, download the full clinical summary.
For a concise visual representation of the 8-year comparative outcomes, download the clinical infographic.
*The RESILIA tissue valve cohort consisted of patients from the COMMENCE aortic trial (n=689)
‡The non-RESILIA tissue valve cohort originated from the Carpentier-Edwards PERIMOUNT Magna Ease valve post-apporval study (n=258)
Clinical data on surgical valves with RESILIA tissue up to 7-year follow-up have been published, with additional follow-up to 10-years in progress.
RESILIA tissue tested against tissue from commercially available bovine pericardial valves from Edwards in a juvenile sheep model. Eur J Cardiothorac Surg. 2021 Jan 29;59(2):434-441. doi: 10.1093/ejcts/ezaa311
COMMENCE Aortic Trial
Methods
- Prospective, multinational, multicenter (n = 27), single-arm, FDA Investigational Device Exemption trial
Full Cohort
- Between January 2013 and March 2016, 689 patients underwent AVR with the Edwards Pericardial Aortic Bioprosthesis with RESILIA tissue (model 11000A)
- Mean age 66.9 ± 11.6 years
- STS risk score 2.0 ± 1.8%
- NYHA Class II and III were 50% and 24%, respectively
- A total of 512 patients completed 5-year follow up
Re-Consented Cohort
- A total of 225 patients were re-consented for extended follow up
- Mean age 65.1 ± 10.9 years
- STS risk score 2.1 ± 2.1%
- NYHA Class II and III were 43% and 19%, respectively
- A total of 195 patients completed 7-year follow up
For a more detailed overview of the COMMENCE trial methodology and results please download the clinical summary.
COMMENCE Aortic Trial 7-Year Clinical Summary

COMMENCE Aortic Trial 7-Year Clinical Summary

COMMENCE Trial Bicuspid Aortic Valve Sub-Analysis
Sub-analysis of COMMENCE aortic trial seven-year outcomes investigating safety outcomes and hemodynamics in patients with bicuspid aortic valves.4
Methods
- Patients underwent echocardiograms annually through 7 years of follow up; these were evaluated by a core laboratory
- All potential safety endpoints adjudicated by an independent Clinical Events Committee and independent echocardiographic core laboratory evaluated hemodynamic performance
- SVD and other safety outcomes defined per "Guidelines for reporting morbidity and mortality after cardiac valve interventions" (Akins et al. 2008)
For a more detailed overview of the COMMENCE trial bicuspid aortic valve sub-analysis methodology and results, download the Infographic.
COMMENCE Trial Bicuspid Aortic Valve Sub-Analysis Infographic

COMMENCE Trial Bicuspid Aortic Valve Sub-Analysis Infographic

COMMENCE Trial Aortic Regurgitation Sub-Analysis
Sub-analysis of the five-year outcomes of the COMMENCE aortic trial investigating clinical and echocardiographic outcomes in patients with pure aortic stenosis compared to those with aortic regurgitation or mixed aortic valve disease5
Methods
- Inclusion criteria involved patients with pure aortic regurgitation (AR) or mixed aortic valve disease (MAVD) defined as moderate or severe regurgitation at baseline with or without aortic stenosis (AS), and patients with pure AS, defined as no regurgitation and mild, moderate, or severe stenosis at baseline
- Evaluations included safety endpoints which were adjudicated by an independent clinical events committee, and hemodynamic performance which was evaluated by an independent echocardiographic core laboratory
For a more detailed overview of the COMMENCE trial five-year aortic regurgitation sub-analysis methodology and results, download the clinical summary.
COMMENCE Trial Aortic Regurgitation Sub-Analysis Clinical Summary

COMMENCE Trial Aortic Regurgitation Sub-Analysis Clinical Summary

COMMENCE Mitral Trial
Mid-term outcomes of the COMMENCE trial investigating mitral valve replacement using a bioprosthesis with a novel tissue6
The COMMENCE trial is an ongoing prospective study to evaluate mitral valve replacement (MVR) using valves with RESILIA tissue.
COMMENCE Mitral Trial 5-Year Clinical Summary
Methods
- Prospective, multi-center (17 sites), single-arm, Investigational Device Exemption trial
- 82 patients underwent MVR with the Edwards Pericardial Mitral Bioprosthesis with RESILIA tissue (model 11000M)
- Median patient age was 70 years with 52.4% of patients aged 70 years or above
- Median follow-up for the study cohort was 5.1 (1.4) years, with a total of 374.2 patient-years in aggregate
- A total of 54 patients completed 5-year follow up
COMMENCE Mitral Trial 5-Year Clinical Summary

EU Feasibility Study
EU Feasibility Study Clinical Summary
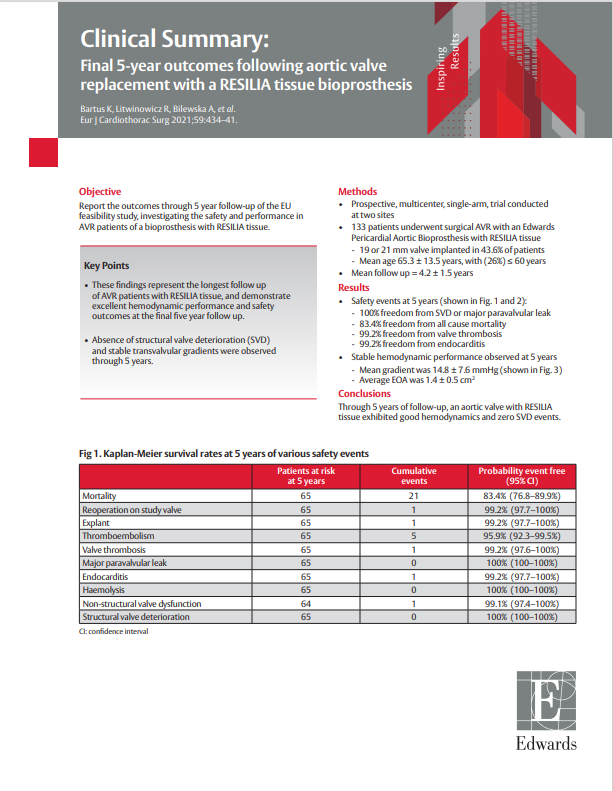
Methods
- Prospective, multicenter, single-arm, trial conducted at two sites
- 133 patients underwent surgical AVR with an Edwards Pericardial Aortic Bioprosthesis with RESILIA tissue
- Mean age 65.3 ± 13.5 years, with (26%) ≤ 60 years
- 19 or 21 mm valve implanted in 43.6% of patients
- Median follow up = 5 years; Total late follow-up time of 565.2 patient years (LPY)
For a more detailed overview of the EU Feasibility Study methodology and results please download the clinical summary.
EU Feasibility Study Clinical Summary

Pannus Formation Animal Study
Pannus Formation Study Clinical Summary

Methods
- This publication reports the outcomes of two independent studies using a juvenile sheep model of mitral valve replacement with bovine pericardial tissue
- In an 8-month study, valves with RESILIA tissue were compared to control valves with XenoLogiX treatment (XLX)
- In a 5-month study, valves with RESILIA tissue were compared to control valves treated with the ThermaFix process (TFX)
- Control valves were commercially available Carpentier-Edwards PERIMOUNT mitral valves, models 6900P, and 7000TFX. Test articles were the same models configured with RESILIA tissue
- Explanted valves were examined macroscopically and histologically. Histological observations were made by an independent pathologist, blinded to group identity
- Independent means of pannus quantification were employed in the two studies
For a more detailed overview of the Pannus Formation Study methodology and results please download the clinical summary.
Pannus Formation Study Clinical Summary

Juvenile Sheep Study
Juvenile Sheep Study Clinical Paper Details

Methods
- 45 juvenile sheep were randomized and either a PERIMOUNT mitral valve (6900P, control group) or the same valve design incorporating the RESILIA tissue preservation technology (test group) was implanted in the mitral position
- All valves were 25 mm
- A transthoracic echocardiography was performed at 1 week and at 8 months postoperatively
- Necropsy was performed at 8 months, and the valves were examined radiographically (soft tissue radiograph), histologically (hematoxylin and eosin and Von Kossa staining), and chemically (calcium content)
For a more detailed overview of the Juvenile Sheep Study methodology and results, please see link to clinical paper details.
Juvenile Sheep Study Clinical Paper Details

Featured Products
Explore our current RESILIA tissue products
References
- Kaneko T, Johnston D, Bavaria JE, et al. Propensity-matched 8-year outcomes following aortic valve replacement with novel versus contemporary bioprostheses. Presented at: Heart Valve Society Annual Scientific Meeting. April 2025; Cairo, Egypt. Abstract available at: https://heartvalvesociety.org/meeting/program/2025/AC22.cgi
- Akins CW, Miller DC, Turina MI, et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. Ann Thorac Surg. 2008;85(4):1490-1495. doi:10.1016/j.athoracsur.2007.12.082
- Beaver T, Bavaria JE, Griffith B, et al. Seven-year outcomes following aortic valve replacement with a novel tissue bioprosthesis. J Thorac Cardiovasc Surg. 2024;168(3):781-791. doi:10.1016/j.jtcvs.2023.09.047
- Salna M, Bavaria JE, Heimansohn D, et al. Seven-Year Results for RESILIA Tissue in Bicuspid Aortic Valve Replacement Patients: Age and Valve Size Considerations. Interdiscip Cardiovasc Thorac Surg. 2025;40(8):ivaf176. doi:10.1093/icvts/ivaf176
- Thourani, VH, Puskas JD, Griffith B, et al. Five-year Comparison of Clinical and Echocardiographic Outcomes of Pure Aortic Stenosis with Pure Aortic Regurgitation or Mixed Aortic Valve Disease in the COMMENCE Trial. JTCVS Open. Sept (2024); https://doi.org/10.1016/j.xjon.2024.08.020.
- Heimansohn DA, Baker C, Rodriguez E, et al. Mid-term outcomes of the COMMENCE trial investigating mitral valve replacement using a bioprosthesis with a novel tissue. JTCVS Open. 2023;15:151-163. Published 2023 Jun 2. doi:10.1016/j.xjon.2023.05.008
- Bartus K, Litwinowicz R, Bilewska A, et al. Final 5-year outcomes following aortic valve replacement with a RESILIA™ tissue bioprosthesis. Eur J Cardiothorac Surg. 2021;59(2):434-441. doi:10.1093/ejcts/ezaa311
- Tod TJ, Gohres RA, Torky M, et al. Influence of Tissue Technology on Pannus Formation on Bioprosthetic Heart Valves. Cardiovasc Eng Technol. 2021;12(4):418-425. doi:10.1007/s13239-021-00530-1
- Flameng W, Hermans H, Verbeken E, Meuris B. A randomized assessment of an advanced tissue preservation technology in the juvenile sheep model. J Thorac Cardiovasc Surg. 2015;149(1):340-345. doi:10.1016/j.jtcvs.2014.09.062
Important safety information
RESILIA Tissue Devices
Indications: INSPIRIS RESILIA Aortic Valve - For use in replacement of native or prosthetic aortic heart valves. KONECT RESILIA Aortic Valved Conduit - For use in replacement of native or prosthetic aortic heart valves and the associated repair or replacement of a damaged or diseased ascending aorta. MITRIS RESILIA Mitral Valve - For use in replacement of native or prosthetic mitral heart valves.
Contraindications: There are no known contraindications with the use of these RESILIA tissue heart valve devices.
Complications and Side Effects: INSPIRIS RESILIA Aortic Valve: Thromboembolism, valve thrombosis, hemorrhage, hemolysis, regurgitation, endocarditis, structural valve deterioration, nonstructural dysfunction, stenosis, arrhythmia, transient ischemic attack/stroke, congestive heart failure, myocardial infarction, any of which could lead to reoperation, explantation, permanent disability, and death. Additional adverse events potentially associated with the use of polyester vascular grafts in the KONECT RESILIA AVC include hemorrhage, thrombosis, graft infection, embolism, aneurysm, pseudoaneurysm, seroma, occlusion (anastomotic intimal hyperplasia), immunological reaction to collagen (shown to be a weak immunogen; infrequent, mild, localized and self-limiting), intimal peel formation, and conduit dilatation. MITRIS RESILIA Mitral Valve - Thromboembolism, valve thrombosis, hemorrhage, hemolysis, regurgitation, endocarditis, structural valve deterioration, nonstructural dysfunction, stenosis, arrhythmia, transient ischemic attack/stroke, congestive heart failure, myocardial infarction, ventricular perforation by stent posts, any of which could lead to reoperation, explantation, permanent disability, and death.
Warnings: INSPIRIS RESILIA Aortic Valve - DO NOT ADJUST THE VALVE DIAMETER BY EXPANDING THE BAND PRIOR TO OR DURING IMPLANTATION OF THE SURGICAL VALVE. The expandable band is not designed to allow for compression or expansion during implantation of the surgical valve. This will cause damage to the valve and may result in aortic incompetence. DO NOT PERFORM STAND-ALONE BALLOON AORTIC VALVULOPLASTY PROCEDURES ON THIS VALVE FOR THE SIZES 19 - 25 mm as this may expand the valve causing aortic incompetence, coronary embolism or annular rupture. Valve-in-valve sizing in the INSPIRIS valve has only been tested with specific Edwards transcatheter heart valves. Use of other transcatheter valves may result in embolization of transcatheter devices anchored within or result in annular rupture.
CAUTION: US law restricts these devices to sale by or on the order of a physician. See instructions for use for full prescribing information.