The Surge

The growing need for durable tissue valves

The 2020 American College of Cardiology and American Heart Association (ACC/AHA) guidelines show an important shift. Determining the best treatment option for a patient starts with shared decision-making and a risk assessment while also looking at age and the patient’s lifestyle needs.
Guidelines support tissue valves for younger patients
The guidelines provide a choice between tissue and mechanical valves for patients between the ages of 50 and 65,1 and tissue valves for patients greater than 65 years old.
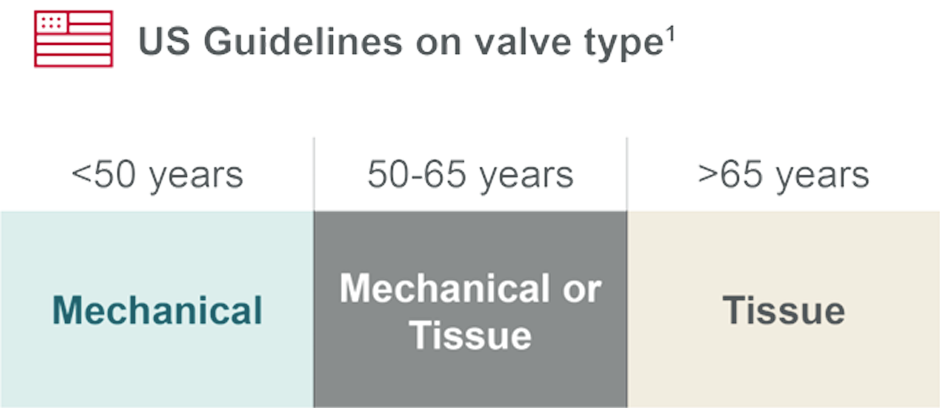
Given these guidelines, there is increased demand for tissue valves with longer durability that offers a choice younger and older patients may need.

For all patients, valve choice should be part of a shared decision-making process that considers the lifetime risks and benefits associated with each option.
Valves with RESILIA tissue: Designed with durability in mind
RESILIA tissue was inspired by the growing need for valves that offer the potential for greater durability and the ability to maintain an active lifestyle.

Tissue with a difference
RESILIA tissue valves have been extensively studied.3 The ongoing COMMENCE aortic trial has allowed us to follow hundreds of patients over many years, enabling us to evaluate the performance and durability of this unique tissue technology.
A growing library of evidence supports RESILIA tissue.
References
- Otto CM, Nishimura RA, Bonow RA, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circ 2021;143(5):e72-e227.
- Flameng W, Hermans H, Verbeken E, et al. A randomized assessment of an advanced tissue preservation technology in the juvenile sheep model. J Thorac Cardiovasc Surg. 2015 Jan;149(1):340-5.
- Beaver T, Bavaria, JE, Griffith B, et al. Seven-year outcomes following aortic valve replacement with a novel tissue bioprosthesis. Presented at the 103rd Annual Meeting of the American Association for Thoracic Surgery, May 2023.
Important Safety Information
RESILIA Tissue Devices
Indications: INSPIRIS RESILIA Aortic Valve - For use in replacement of native or prosthetic aortic heart valves. KONECT RESILIA Aortic Valved Conduit - For use in replacement of native or prosthetic aortic heart valves and the associated repair or replacement of a damaged or diseased ascending aorta. MITRIS RESILIA Mitral Valve - For use in replacement of native or prosthetic mitral heart valves.
Contraindications: There are no known contraindications with the use of these RESILIA tissue heart valve devices.
Complications and Side Effects: INSPIRIS RESILIA Aortic Valve - Thromboembolism, valve thrombosis, hemorrhage, hemolysis, regurgitation, endocarditis, structural valve deterioration, nonstructural dysfunction, stenosis, arrhythmia, transient ischemic attack/stroke, congestive heart failure, myocardial infarction, any of which could lead to reoperation, explantation, permanent disability, and death. Additional adverse events potentially associated with the use of polyester vascular grafts in the KONECT RESILIA AVC include hemorrhage, thrombosis, graft infection, embolism, aneurysm, pseudoaneurysm, seroma, occlusion (anastomotic intimal hyperplasia), immunological reaction to collagen (shown to be a weak immunogen; infrequent, mild, localized and self-limiting), intimal peel formation, and conduit dilatation. MITRIS RESILIA Mitral Valve - Thromboembolism, valve thrombosis, hemorrhage, hemolysis, regurgitation, endocarditis, structural valve deterioration, nonstructural dysfunction, stenosis, arrhythmia, transient ischemic attack/stroke, congestive heart failure, myocardial infarction, ventricular perforation by stent posts, any of which could lead to reoperation, explantation, permanent disability, and death.
Warnings: INSPIRIS RESILIA Aortic Valve - DO NOT ADJUST THE VALVE DIAMETER BY EXPANDING THE BAND PRIOR TO OR DURING IMPLANTATION OF THE SURGICAL VALVE. The expandable band is not designed to allow for compression or expansion during implantation of the surgical valve. This will cause damage to the valve and may result in aortic incompetence. DO NOT PERFORM STAND-ALONE BALLOON AORTIC VALVULOPLASTY PROCEDURES ON THIS VALVE FOR THE SIZES 19 – 25 mm as this may expand the valve causing aortic incompetence, coronary embolism or annular rupture. Valve-in-valve sizing in the INSPIRIS valve has only been tested with specific Edwards transcatheter heart valves. Use of other transcatheter valves may result in embolization of transcatheter devices anchored within or result in annular rupture.
CAUTION: Federal (USA) law restricts these devices to sale by or on the order of a physician. See instructions for use for full prescribing information.
Edwards, Edwards Lifesciences, the stylized E logo, COMMENCE, INSPIRIS, INSPIRIS RESILIA, KONECT, KONECT RESILIA, MITRIS, MITRIS RESILIA, and RESILIA are trademarks or service marks of Edwards Lifesciences Corporation or its affiliates. All other trademarks are the property of their respective owners.
© 2023 Edwards Lifesciences Corporation. All rights reserved. PP--US-8075 v1.0
Edwards Lifesciences • One Edwards Way, Irvine CA 92614 USA • edwards.com

