The ALLIANCE trial


About the ALLIANCE trial

The ALLIANCE trial will evaluate if the SAPIEN X4 transcatheter heart valve (THV) is beneficial for patients with symptomatic, severe, calcific aortic stenosis. Talk to your doctor to determine if this trial is right for you.
Contact our Patient Support Center to find a participating hospital near you.
What is symptomatic, severe, calcific aortic stenosis?
Your heart has four valves, that open and shut like doors, to help pump blood through your body. One of the four valves is called the aortic valve. The tissue from the valve, that opens and shuts like doors, are called leaflets. These leaflets can become stiff. The stiffness causes the opening of the valve to become smaller. This means the valve cannot fully open and shut like it should. As the opening becomes smaller, it makes it harder for the heart to pump blood, which can affect your health. This condition is called aortic stenosis.
Aortic stenosis is a progressive disease, which means it will get worse over time. Because of this, doctors will typically measure it as mild, moderate, or severe aortic stenosis. The stage of aortic stenosis depends on how damaged your aortic valve is. Severe aortic stenosis is the advanced case of aortic stenosis.
Disease progression in the valve leaflets in aortic stenosis

About the study device

The Edwards SAPIEN X4 valve is an experimental, artificial heart valve that is made to replace your diseased aortic heart valve. Each valve consists of a metal frame with knitted fabric covering some of the frame. The frame has leaflets attached that are made of cow tissue and which open and close fully to direct the flow of blood through your heart the way your normal valve would if it were healthy. The device is implanted through the transcatheter aortic valve replacement (TAVR) procedure, which is minimally invasive and uses a catheter to implant a new valve through a small incision in your groin.

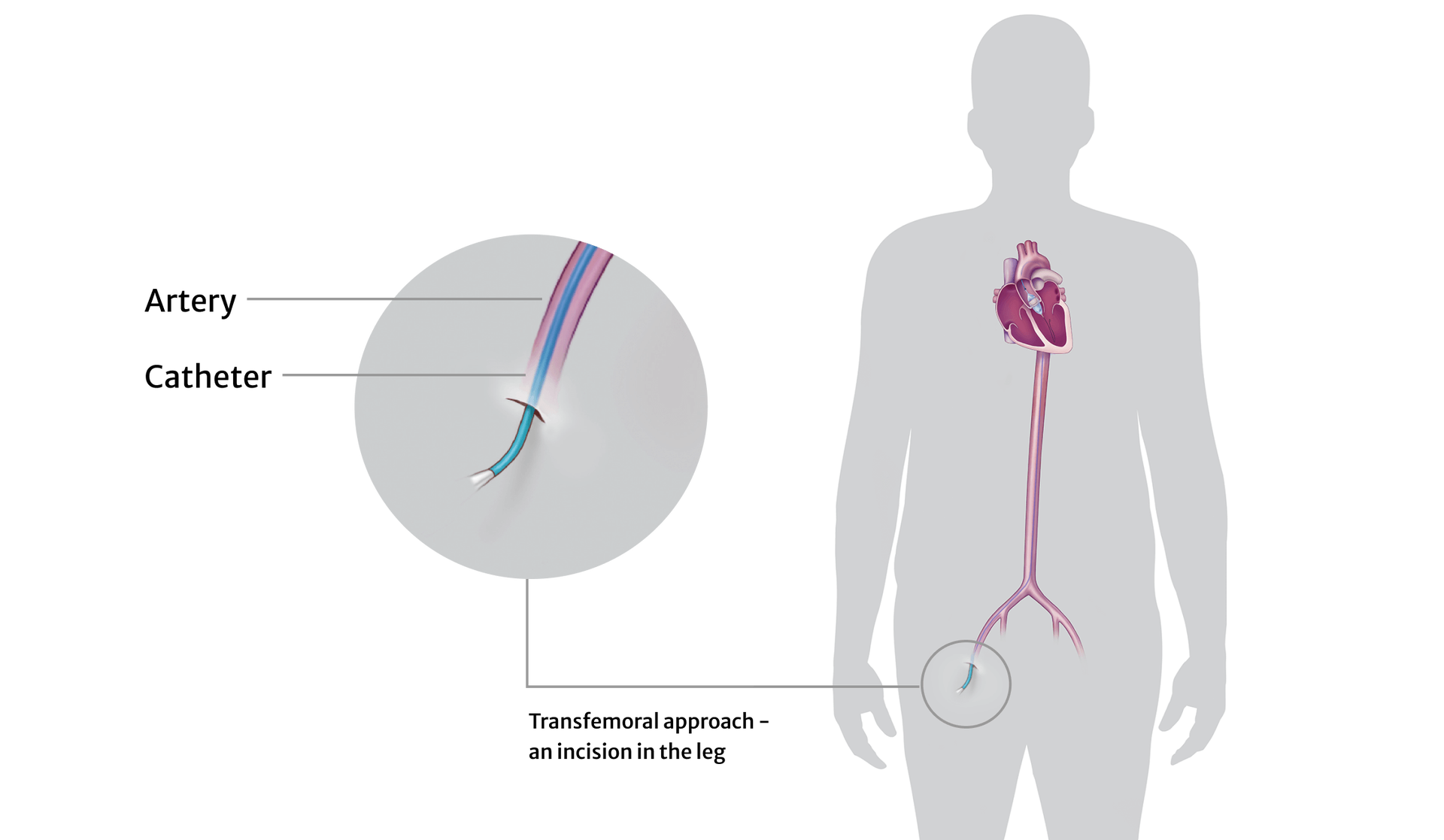
If you agree to participate and are enrolled into this research study, your doctor will use the SAPIEN X4 transcatheter heart valve system to replace your valve. The study doctor has received extensive training on this SAPIEN X4 system by the manufacturer of the study device, Edwards Lifesciences. By participating in this trial, you may be able to help your doctors as well as helping others like yourself.
Eligibility criteria
- Severe, calcific aortic stenosis
- Native aortic annulus size suitable for SAPIEN X4 transcatheter heart valve
- New York Heart Association functional class ≥ II
- Full inclusion and exclusion criteria are detailed in the clinical protocol
Frequently Asked Questions
Learn more about the procedure

We’re here for you
We are committed to providing the highest levels of customer service to help our patients improve their quality of life. For any questions, please contact the Edwards Patient Support Center.
For details about the trial, visit NCT05172960 at ClinicalTrials.gov.
Give us a call
Send us an email
CAUTION: Investigational device. Limited by Federal (United States) law to investigational use.
