Stenosis
when your aortic bioprosthetic valve narrows and does not open completely
Now enrolling



The ALLIANCE aortic valve-in-valve (AViV) trial will evaluate if the SAPIEN X4 transcatheter heart valve (THV) is beneficial for patients with a failing aortic bioprosthetic valve (a man-made valve containing animal tissue). Talk to your doctor to determine if this trial is right for you.
Contact our Patient Support Center to find a participating hospital near you.
Your original aortic valve has already been replaced with a bioprosthetic valve. Over time, there are two common problems that can cause your bioprosthetic heart valve to fail:
when your aortic bioprosthetic valve narrows and does not open completely
when your surgical bioprosthetic valve does not close completely and blood leaks backwards
Your existing surgical bioprosthetic aortic valve is failing and needs to be replaced
You fall in the high or greater risk category for repeat open heart surgery

The Edwards SAPIEN X4 THV is an experimental artificial heart valve that is designed to replace your failing bioprosthetic aortic heart valve. The valve consists of a metal frame with knitted fabric covering some of the frame, and it has leaflets made of cow tissue that open and close fully to direct the flow of blood properly through your heart. The device is implanted through the transcatheter aortic valve replacement (TAVR) procedure, which is minimally invasive and uses a catheter to implant a new valve through a small incision in your groin.

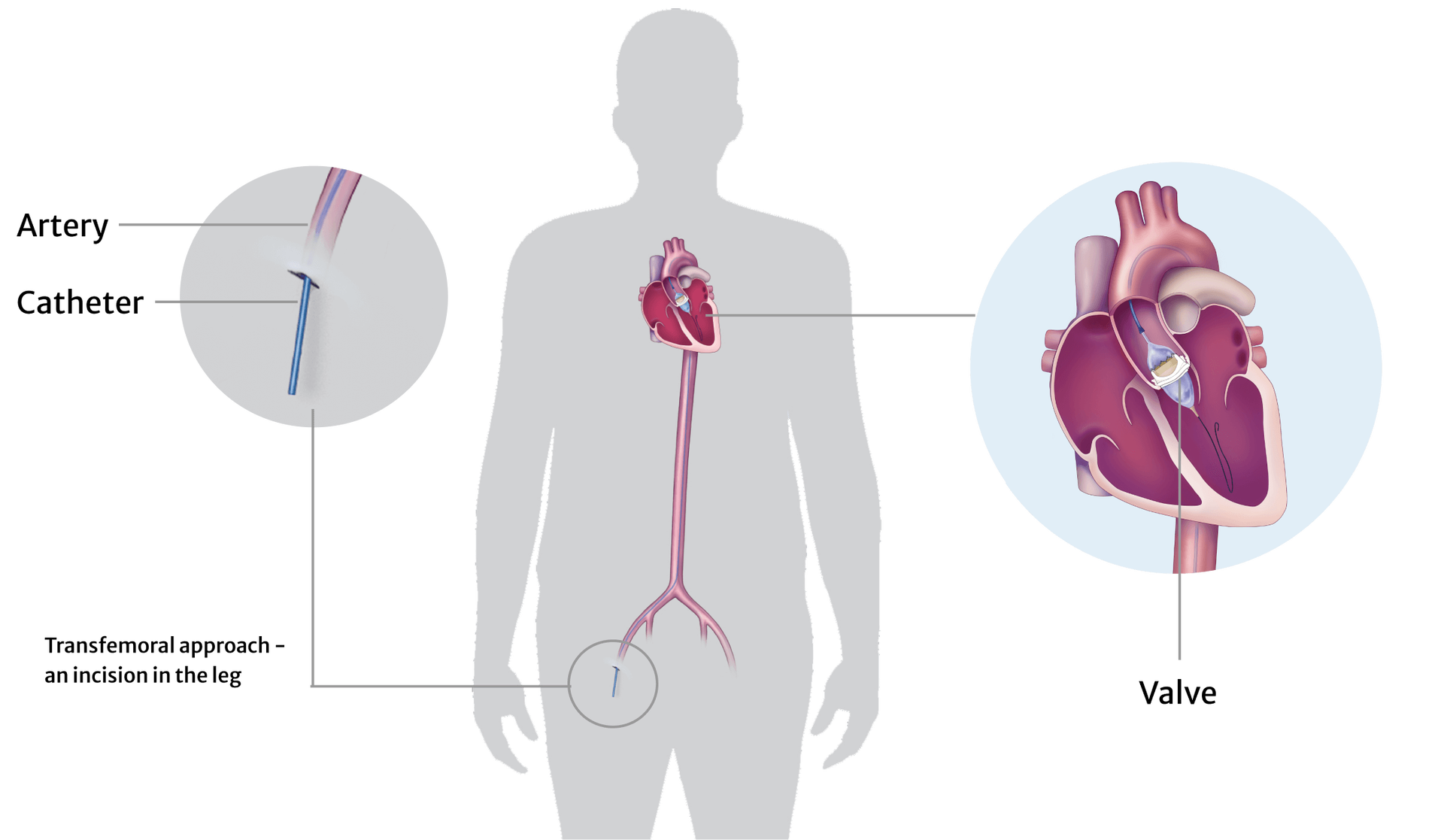
If you agree to participate and are enrolled into this research study, your doctor will use the SAPIEN X4 transcatheter heart valve system to replace your failing bioprosthetic aortic heart valve. The study doctor has received extensive training on this SAPIEN X4 system by the manufacturer of the study device, Edwards Lifesciences. By participating in this trial, you may be able to help your doctors while helping others like yourself.
Full inclusion and exclusion criteria are detailed in the clinical protocol

We are committed to providing the highest levels of customer service to help our patients improve their quality of life. For any questions, please contact the Edwards Patient Support Center.
For details about the trial, visit NCT05172973 at ClinicalTrials.gov.
Give us a call
Send us an email
CAUTION: Investigational device. Limited by Federal (United States) law to investigational use.