Asymptomatic
Negative stress test* OR confirmation via medical history
This trial is no longer enrolling patients



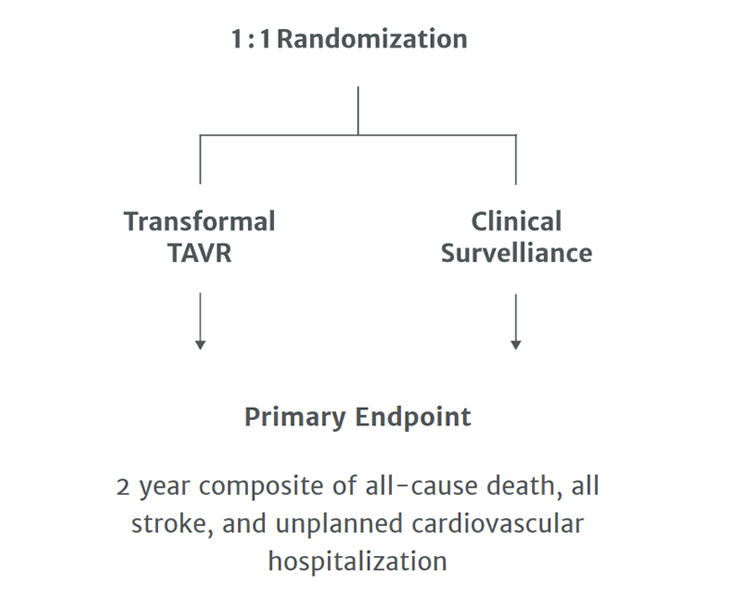
To establish the safety and effectiveness of the Edwards SAPIEN 3/ SAPIEN 3 Ultra transcatheter heart valve (THV) compared with clinical surveillance (CS) in asymptomatic patients with severe, calcific aortic stenosis
Recommended treatment for patients with asymptomatic stenosis (AS) includes following patients clinically until they become symptomatic, left ventricular dysfunction develops, or cardiac surgery is recommended for other reasons.

Whether early intervention before symptoms develop will improve outcomes remains unknown and has never been studied in a randomized trial.
Patients will undergo a treadmill stress test to determine if they are asymptomatic with severe aortic stenosis.

Negative stress test* OR confirmation via medical history

Positive stress test*
*For patients who can perform treadmill stress test



*For patients who can perform treadmill stress test



We are committed to providing the highest levels of customer service to help our patients improve their quality of life. For any questions, please contact the Edwards Patient Support Center. For details about the trial, visit NCT03042104 at Clinical Trials.gov.
Give us a call
Send us an email
The Edwards SAPIEN 3 / Edwards SAPIEN 3 Ultra transcatheter heart valve is an investigational device when used in asymptomatic patients. Limited by Federal (USA) law to investigational use only. These devices are not available for marketing or commercial sale in the United States for asymptomatic patients.