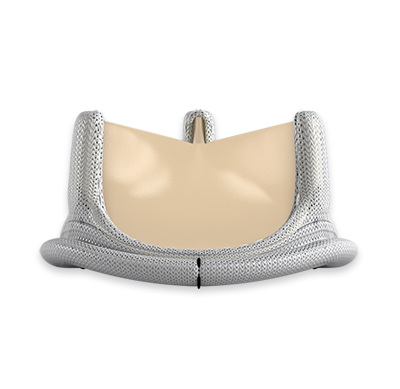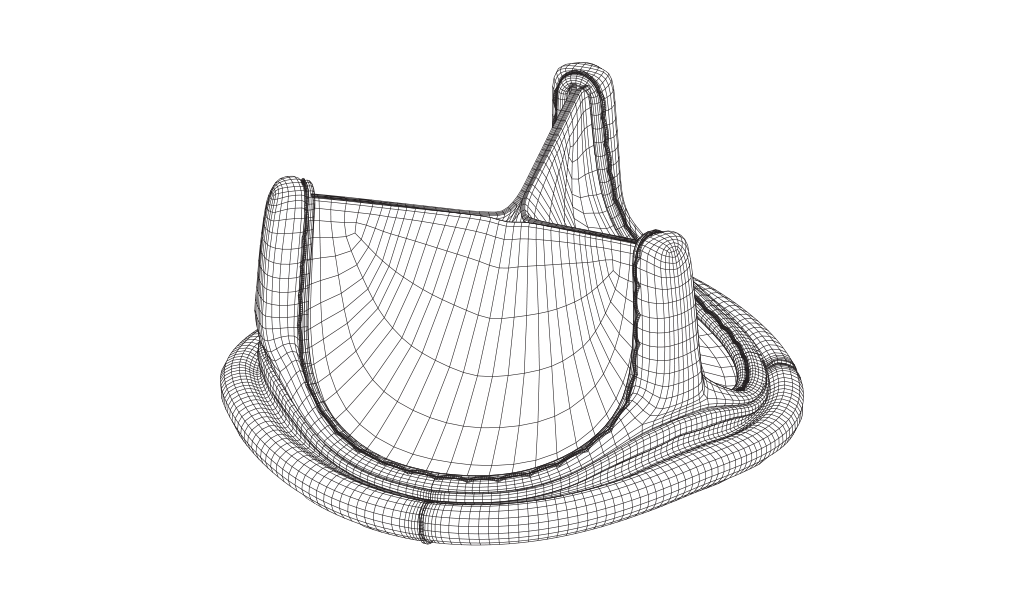
Mathematically modeled, bioengineered design
Optimized for hemodynamics, durability and implantability

Built upon the Carpentier-Edwards PERIMOUNT bioprosthesis design.

The Carpentier-Edwards PERIMOUNT Magna Ease aortic valve is part of the proven family of PERIMOUNT surgical valves.
Backed by over 20 years of published clinical studies for proven longevity and demonstrated hemodynamic stability, the Carpentier-Edwards PERIMOUNT Magna Ease aortic valve combines a tested design with the benefits of pericardial tissue to deliver the potential for on-going durability.

Optimized for hemodynamics, durability and implantability

Absorbs energy to reduce leaflet stress

Matched for thickness and elasticity to optimize stress distribution
The Magna Ease valves demonstrated excellent durability after the equivalent of 25 years in simulated in vitro wear, with hydrodynamic performance similar to that of a new valve.
More long-term clinical study publications than any other bioprosthetic aortic valve.


* Freedom from explant/prosthesis replacement/reoperation due to SVD
The PERIMOUNT Magna Ease valve offers many key design features that enhance the valve’s ease of implant.
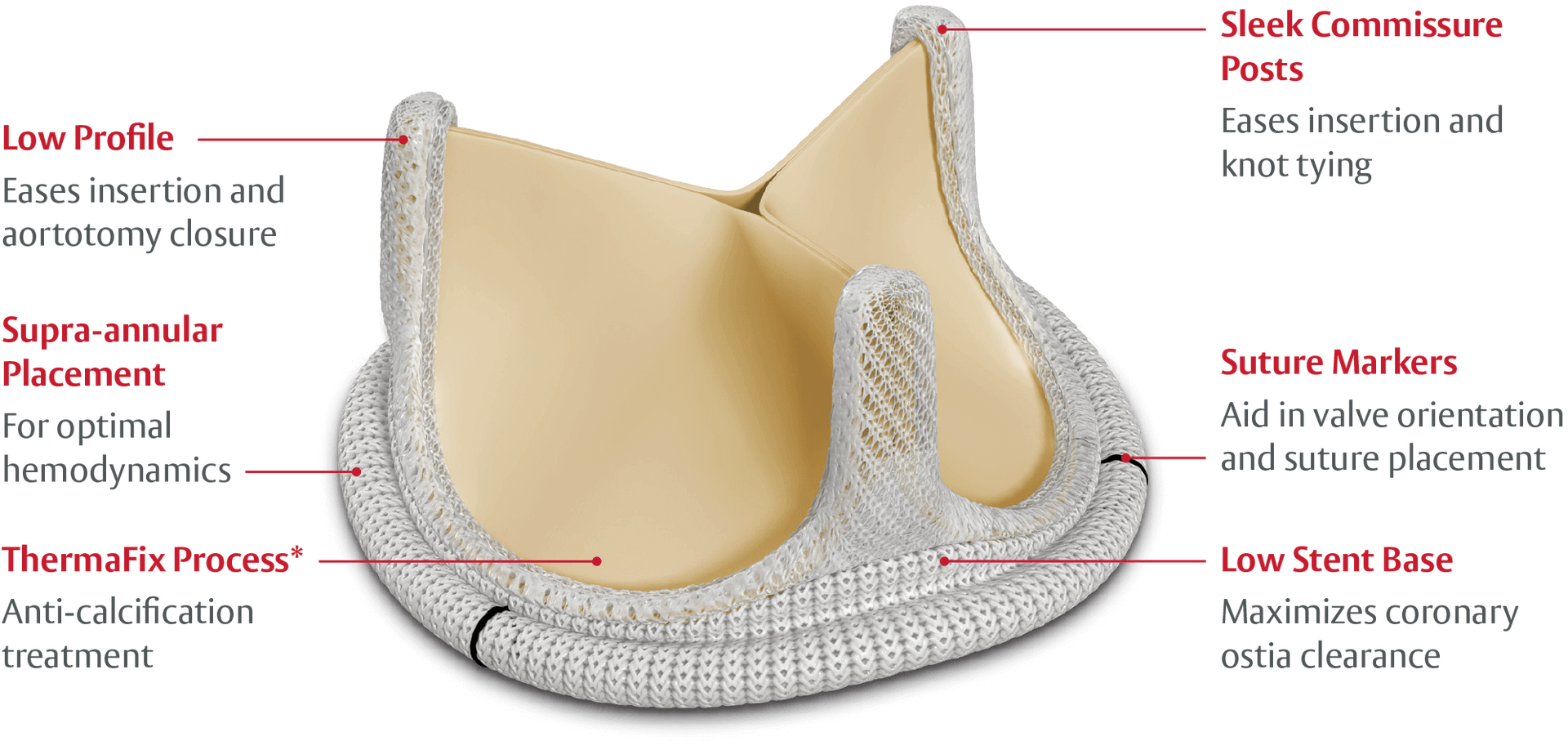
*No clinical data are available that evaluate the long-term impact of the Carpentier-Edwards ThermaFix issue process in patients
Excellent EOAs and low gradients based on the proven PERIMOUNT valve design.15

The Carpentier-Edwards PERIMOUNT Magna Ease pericardial bioprosthesis is intended for use in patients whose aortic valvular disease is sufficiently advanced to warrant replacement of their natural valve with a prosthetic one. It is also intended for use in patients with a previously implanted aortic valve prosthesis that is no longer functioning adequately and requires replacement. In the latter case, the previously implanted prosthesis is surgically excised and replaced by the replacement prosthesis.
*No clinical data are available that evaluate the long-term impact of the Carpentier-Edwards ThermaFix issue process in patients
Carpentier-Edwards PERIMOUNT Aortic Bioprostheses
Indications: For use in patients whose aortic valvular disease warrants replacement of their natural or previously placed prosthetic valve.
Contraindications: Do not use if surgeon believes it would be contrary to the patient’s best interests.
Complications and side effects: Stenosis, regurgitation, endocarditis, hemolysis, thromboembolism, valve thrombosis, nonstructural dysfunction, structural valve deterioration, anemia, arrhythmia, hemorrhage, transient ischemic attack/stroke, congestive heart failure, myocardial infarction, angina, any of which could lead to reoperation, explantation, permanent disability, and death.
CAUTION: Federal (USA) law restricts these devices to sale by or on the order of a physician. See instructions for use for full prescribing information.
We are committed to providing your institution, clinicians and staff with the highest levels of customer service and support to ensure seamless product implementation and ongoing use, including:
24/7 Technical support
For product information and orders