
The Surge

Performing more concomitant procedures: Are you aware of the New DRG 212?

The Centers for Medicare and Medicaid Services (CMS) developed the Medicare Severity Diagnosis Related Groups (MS-DRG) system to categorize patients with similar diagnoses and stratify them based on a hospital’s case-mix complexity. MS-DRGs are updated yearly to reflect changes in the healthcare landscape and to ensure they are maintaining the highest level of quality and efficiency in patient care. 1
This year’s update includes the addition of a new MS-DRG that features important considerations for surgery teams performing concomitant valve procedures. 2
Higher rates for concomitant cardiac procedures
As of October 1, 2023, MS-DRG 212 went into effect, which supports concomitant aortic and mitral procedures along with a third procedure during an index operation with a base rate of $75,412 for fiscal year (FY) 2024.
This higher rate reflects the complexity and increased resource use of performing concomitant procedures.2
Medicare FY 2024 national average base payment rates2
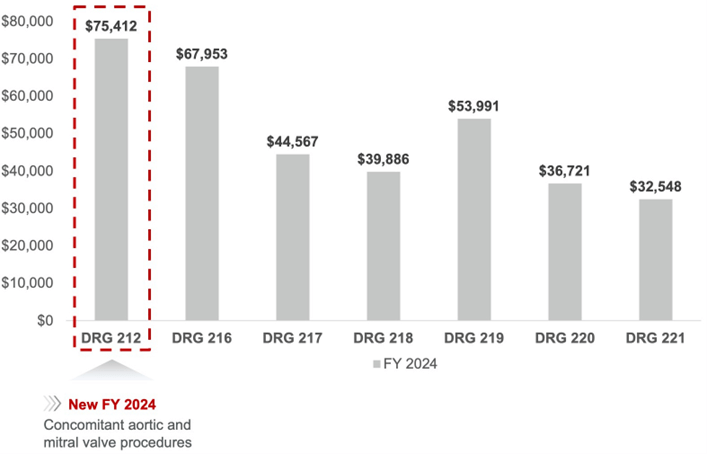
The DRGs are segmented based on documented patient diagnoses of comorbidities and complications. Therefore, accurate documentation of a patient’s diagnosis is vital as it determines the assignment to Diagnosis Related Groups (DRGs), thereby accurately reflecting the case-mix complexity and ensuring proper reimbursement.
How is MS-DRG 212 for concomitant valve procedures defined?
Concomitant aortic and mitral valve procedures can now be tracked to MS-DRG 212. For example, if a patient undergoing aortic and mitral valve repair/replacement procedure also undergoes a coronary artery bypass graft surgery (CABG) and/or open surgical ablation, the case will be assigned to the newly created concomitant procedures MS-DRG 212.3
For a case to be assigned to MS-DRG 212, three conditions have to be met. The case should include a procedure from each of the groups below:
Concomitant procedures assigned to MS-DRG 212
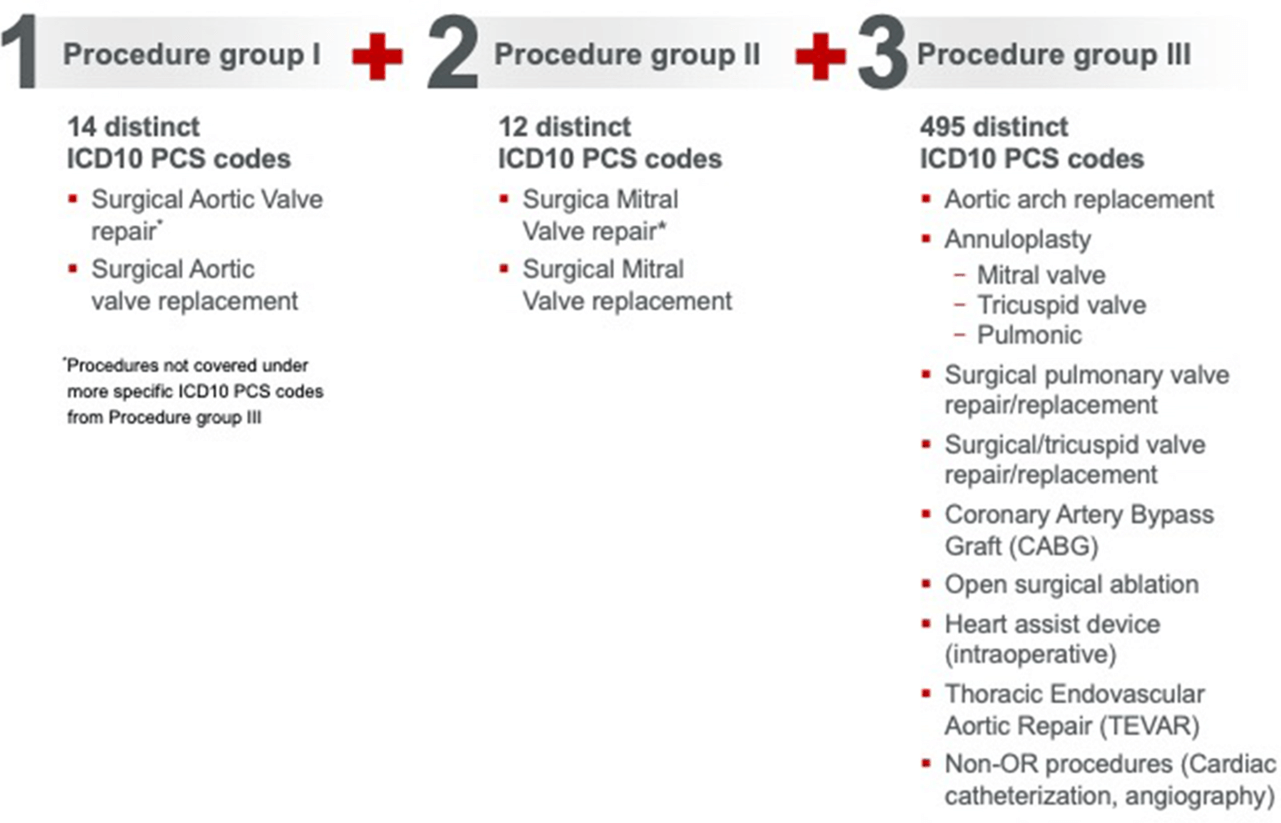
Bentall procedures with concomitant mitral valve repair/replacement will also be paid under MS-DRG 212.
The reimbursement landscape is evolving, and rates are adapting to reflect the complexity of today’s patients and cases. Medicare’s recent guidance ensures that reimbursement updates accurately mirror the evolving treatment landscape, providing necessary treatment for patients needing simultaneous/concomitant valve repair and replacement during the primary procedure. With these trends in mind, innovative therapies that meet patients’ clinical needs and post-procedure wants can be considered more often.
Get comprehensive information on coding and reimbursement for surgical valve procedures
Know the top comorbidities and complications that accompany common surgical valve procedures—download these pocket cards:
Important- Please Note: This information is provided as a general resource and is not intended to constitute medical advice or in any way replace the independent medical judgment of a trained and licensed physician with respect to any individual patient needs or circumstances. Coverage, reimbursement, and health economics information provided by Edwards is gathered from third-party sources and presented for illustrative purposes only. This information does not constitute reimbursement or legal advice, and Edwards makes no representation or warranty regarding this information or its completeness, accuracy, or timeliness. Laws, regulations, and payer policies concerning reimbursement are complex and change frequently; service providers are responsible for all decisions relating to coding and reimbursement submissions.
References
- Centers for Medicare & Medicaid Services. Design and Development of the Diagnosis Related Group. https://www.cms.gov/icd10m/version37-fullcode-cms/fullcode_cms/Design_and_development_of_the_Diagnosis_Related_Group_(DRGs).pdf. Accessed September 24, 2024
- FY ‘24 IPPS Final rule.
- ICD-10-CM/PCS MS-DRG v41.0 Definitions Manual https://www.cms.gov/icd10m/FY2024-nprmversion41.0fullcodecms/fullcode_cms/P0008.html. Accessed December 5, 2023
Edwards, Edwards Lifesciences, and the stylized E logo are trademarks of Edwards Lifesciences Corporation or its affiliates. All other trademarks are the property of their respective owners.
© 2024 Edwards Lifesciences Corporation. All rights reserved. PP--US-9741 v1.0
Edwards Lifesciences • One Edwards Way, Irvine CA 92614 USA • edwards.com


