The Surge

Recent clinical study offers perspective on mitral valve replacement age guidelines

Recent clinical study offers perspective on mitral valve replacement age guidelines
The American Heart Association guidelines suggest that bioprosthetic mitral valves are appropriate for patients over 65. However, the opportunity to avoid lifelong anticoagulation and the associated risks with mechanical mitral valve replacement may be of interest to younger patients as well.1
According to promising results from a recent study between two large university-based cardiac surgery programs, bovine pericardial mitral valve replacement may be a durable option for these younger patients.1
Addressing a need
In a retrospective study, Romano et al, evaluate the late outcomes of bioprosthetic valves in MVR patients with an emphasis on patients under 65 years old, an understudied population.
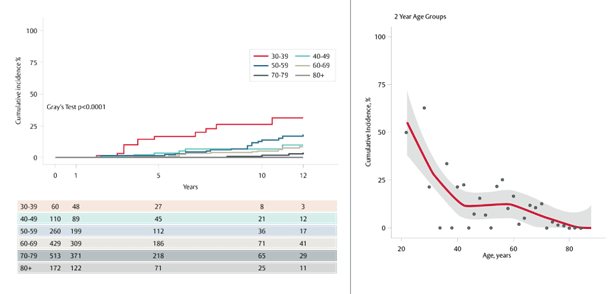
This study sought to determine the durability of contemporary mitral bioprosthetic valves in 1,544 patients (mean age 66±13 years) between 2004 to 2020, of which 652 (42%) were < 65 years of age.
Durability outcomes involving structural valve deterioration (SVD) were compared by age decile. Durability was defined as freedom from the need for reintervention by reoperation or a transcatheter valve-in-valve technique.
Promising results
Bovine pericardial mitral valves demonstrated a low rate of SVD—even in patients who were less than 65 years of age.
The cumulative incidence of mitral valve reinterventions performed for SVD among all patients was:
- 6.2% at 10 years
- 9.0% at 12 years
The study authors were surprised by the relatively flat curve for SVD in patients ages 40 to 69, and the risk of reintervention for SVD (12.4%) at 12 years, which was lower than previous reports, and did not differ significantly among those years (P = 0.1).
The authors also noted that the risk of mortality with reoperation was low (5.6%).
Looking ahead
With observed improved bioprosthetic mitral valve durability and the lower risk for potential late reintervention, the study authors suggest that current guidelines should be reassessed, and that updating the shared decision-making conversation should be considered.1
The authors noted that anti-calcification treatment changed during this time, which may have influenced durability.
References
- Romano M, McCarthy PM, Baldridge AS, et al. Should Mitral Valve Replacement Age Guidelines Be Lowered Due to Better Bioprosthetic Mitral Valve Durability? J Thorac Cardiovasc Surg. 2023. Oct 13:S0022- 5223(23)00968-6. doi: 10.1016/j.jtcvs.2023.10.012.
Important Safety Information
Carpentier-Edwards PERIMOUNT Mitral Bioprostheses
Indications: For use in patients whose mitral valvular disease warrants replacement of their natural or previously placed prosthetic mitral valve.
Contraindications: Do not use if surgeon believes such would be contrary to the patient’s best interests.
Complications and Side Effects: Stenosis, regurgitation, endocarditis, hemolysis, thromboembolism, valve thrombosis, nonstructural dysfunction, structural valve deterioration, anemia, arrhythmia, hemorrhage, transient ischemic attack/ stroke, congestive heart failure, myocardial infarction, angina, ventricular perforation by stent posts any of which could lead to reoperation, explantation, permanent disability, and death.3
CAUTION: Federal (USA) law restricts these devices to sale by or on the order of a physician. See instructions for use for full prescribing information.
Edwards, Edwards Lifesciences, the stylized E logo, Carpentier-Edwards, CarpentierEdwards PERIMOUNT, PERI, and PERIMOUNT are trademarks of Edwards Lifesciences Corporation or its affiliates. All other trademarks are the property of their respective owners.
© 2024 Edwards Lifesciences Corporation. All rights reserved. PP--US-8889 v1.0
Edwards Lifesciences • One Edwards Way, Irvine CA 92614 USA • edwards.com

