The Surge

Long-run savings with RESILIA tissue bioprosthetic valves1

When deciding whether to use a tissue valve or mechanical valve for surgical aortic valve replacement (SAVR), key factors include expected durability, the potential for reoperation, the patient’s expected lifespan after the operation, and other lifestyle preferences.
While studies have identified health outcomes associated with tissue and mechanical valves for SAVR, only recently have researchers investigated the expected trajectory of US health system costs associated with the different valve types.
One study published in 2023 quantifies the expected 15-year run savings when using a RESILIA tissue valve relative to a mechanical valve for SAVR.

About the economic evaluation study
In this study, two SAVR cohort models (tissue vs. mechanical) of 10,000 patients were used to estimate disease progression over a 15-year period. The first 5 years for RESILIA tissue data was based on results from the COMMENCE trial, and the additional 10 years relied on weighted data from three primary legacy long-term tissue valve studies.1-4
Savings potential with RESILIA tissue
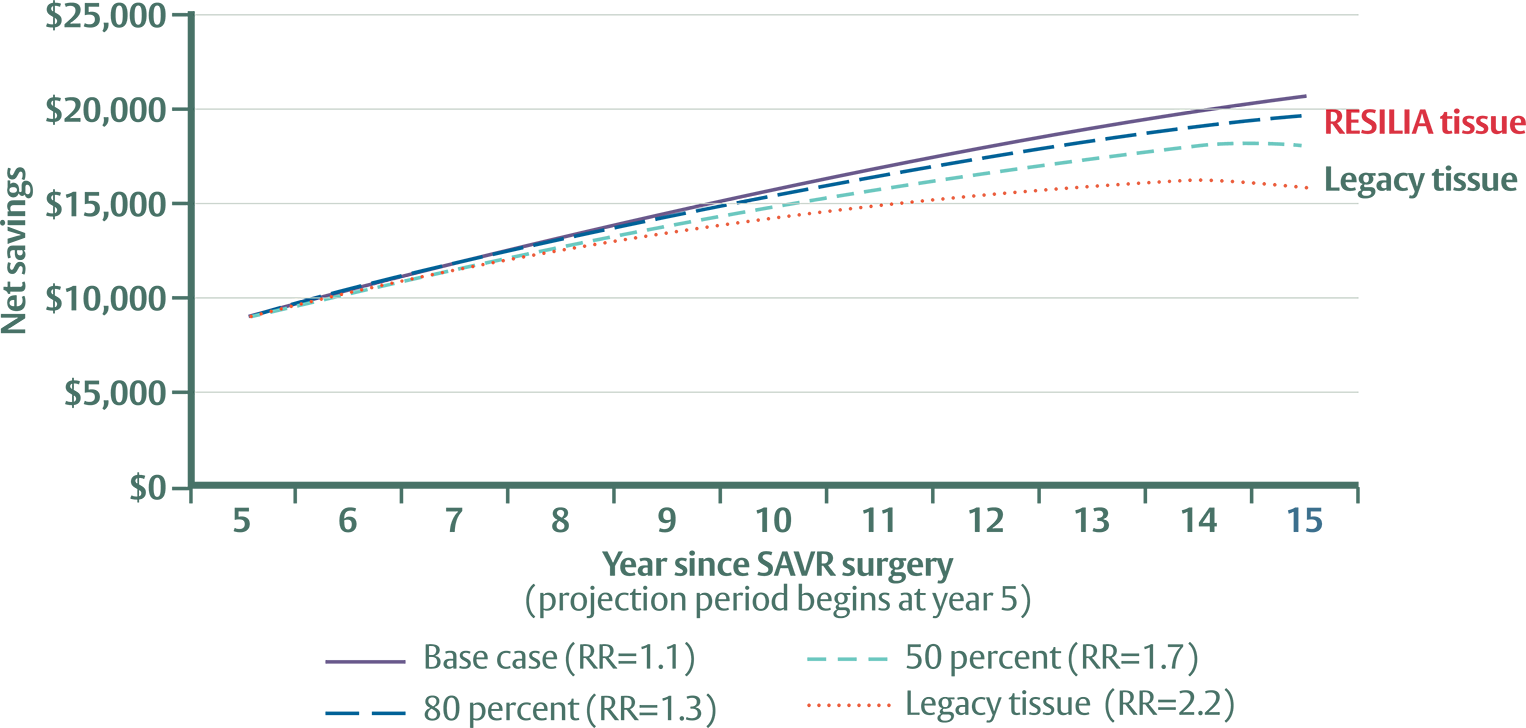
Based on the data evaluated, the discounted cumulative savings associated with RESILIA tissue valves is significant:
- $8,872 at 5 years
- $20,498 projected at 15 years
RESILIA tissue valves are anticipated to accrue approximately 30% to 50% larger savings than legacy tissue valves when compared to mechanical valves.
The RESILIA tissue difference*
RESILIA tissue is made with advanced anti-calcification technology, designed for extended durability.5 In addition to this unique benefit, this study demonstrates the possible savings with RESILIA tissue.
SAVR with RESILIA tissue valves may further reduce future health system expenditures relative to mechanical valves due to the potential lower rate of reoperation compared to legacy tissue technology.
*No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients. RESILIA tissue tested against tissue from commercially-available bovine pericardial valves from Edwards in a juvenile sheep model. Flameng, et al. J Thorac Cardiovasc Surg 2015;149:340-5.
Want to see more data from the economic evaluation study?
Related Articles
References
- Keuffel EL, Reifenberger M, Marfo G, Nguyen TC. Long-run savings associated with surgical aortic valve replacement using a RESILIA tissue bioprosthetic valve versus a mechanical valve. J Med Econ. 2023;26(1):120-127.
- Bourguignon T, Bouquiaux-Stablo A-L, Candolfi P, et al. Very long-term outcomes of the Carpentier-Edwards perimount valve in aortic position. Ann Thorac Surg. 2015;99(3):831–837.
- Bourguignon T, El Khoury R, Candolfi P, et al. Very long-term outcomes of the Carpentier-Edwards perimount aortic valve in patients aged 60 or younger. Ann Thorac Surg. 2015;100(3):853–859.
- Bourguignon T, Lhommet P, El Khoury R, et al. Very long-term outcomes of the Carpentier-Edwards perimount aortic valve in patients aged 50–65 years. Eur J Cardiothorac Surg. 2016;49(5):1462–1468.
- Flameng W, Hermans H, Verbeken E, et al. A randomized assessment of an advanced tissue preservation technology in the juvenile sheep model. J Thorac Cardiovasc Surg. 2015;149(1):340–345.
Important Safety Information
RESILIA Tissue Devices
Indications: INSPIRIS RESILIA Aortic Valve - For use in replacement of native or prosthetic aortic heart valves. KONECT RESILIA Aortic Valved Conduit - For use in replacement of native or prosthetic aortic heart valves and the associated repair or replacement of a damaged or diseased ascending aorta. MITRIS RESILIA Mitral Valve - For use in replacement of native or prosthetic mitral heart valves.
Contraindications: There are no known contraindications with the use of these RESILIA tissue heart valve devices.
Complications and Side Effects: INSPIRIS RESILIA Aortic Valve - Thromboembolism, valve thrombosis, hemorrhage, hemolysis, regurgitation, endocarditis, structural valve deterioration, nonstructural dysfunction, stenosis, arrhythmia, transient ischemic attack/stroke, congestive heart failure, myocardial infarction, any of which could lead to reoperation, explantation, permanent disability, and death. Additional adverse events potentially associated with the use of polyester vascular grafts in the KONECT RESILIA AVC include hemorrhage, thrombosis, graft infection, embolism, aneurysm, pseudoaneurysm, seroma, occlusion (anastomotic intimal hyperplasia), immunological reaction to collagen (shown to be a weak immunogen; infrequent, mild, localized and self-limiting), intimal peel formation, and conduit dilatation. MITRIS RESILIA Mitral Valve - Thromboembolism, valve thrombosis, hemorrhage, hemolysis, regurgitation, endocarditis, structural valve deterioration, nonstructural dysfunction, stenosis, arrhythmia, transient ischemic attack/stroke, congestive heart failure, myocardial infarction, ventricular perforation by stent posts, any of which could lead to reoperation, explantation, permanent disability, and death.
Warnings: INSPIRIS RESILIA Aortic Valve - DO NOT ADJUST THE VALVE DIAMETER BY EXPANDING THE BAND PRIOR TO OR DURING IMPLANTATION OF THE SURGICAL VALVE. The expandable band is not designed to allow for compression or expansion during implantation of the surgical valve. This will cause damage to the valve and may result in aortic incompetence. DO NOT PERFORM STAND-ALONE BALLOON AORTIC VALVULOPLASTY PROCEDURES ON THIS VALVE FOR THE SIZES 19 – 25 mm as this may expand the valve causing aortic incompetence, coronary embolism or annular rupture. Valve-in-valve sizing in the INSPIRIS valve has only been tested with specific Edwards transcatheter heart valves. Use of other transcatheter valves may result in embolization of transcatheter devices anchored within or result in annular rupture.
CAUTION: Federal (USA) law restricts these devices to sale by or on the order of a physician. See instructions for use for full prescribing information.
Edwards, Edwards Lifesciences, the stylized E logo, COMMENCE, INSPIRIS, INSPIRIS RESILIA, KONECT, KONECT RESILIA, MITRIS, MITRIS RESILIA, and RESILIA are trademarks or service marks of Edwards Lifesciences Corporation or its affiliates. All other trademarks are the property of their respective owners.
© 2023 Edwards Lifesciences Corporation. All rights reserved. PP--US-8514 v1.0
Edwards Lifesciences • One Edwards Way, Irvine CA 92614 USA • edwards.com


