A new publication examines the KONECT RESILIA aortic valved conduit for aortic root replacement
In-Human Implantation of a Novel Biologic Valved Conduit for Aortic Root Replacement by DeRoo, et al.
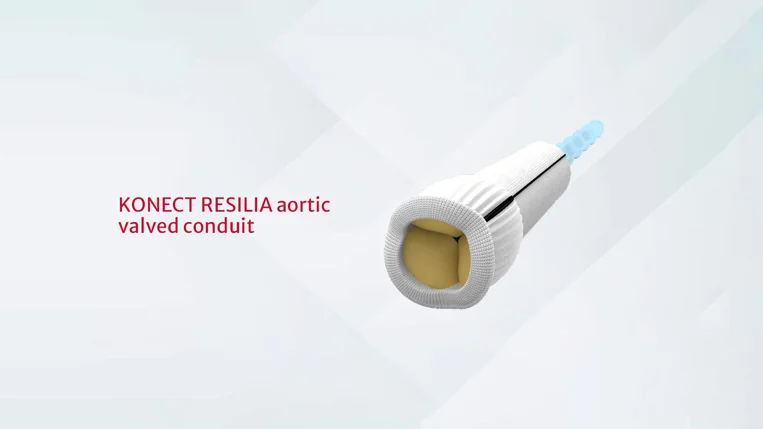
Aortic root replacement is a common but complex cardiac surgical procedure. The KONECT RESILIA aortic valved conduit (AVC) is the first pre-assembled, ready-to-implant* valved conduit with RESILIA tissue. It is built on the proven performance of both the Carpentier–Edwards PERIMOUNT valve platform and the Gelweave Valsalva graft. The pre-assembled KONECT RESILIA aortic valved conduit intuitively eliminates procedural steps†, which is especially important in emergency cases.
The authors of the study reported on three aortic root replacement patients receiving the KONECT RESILIA AVC. All patients were transported to the cardiothoracic ICU after their procedures, discharged after a median stay of 5 days, and seen at a 6-month follow-up. None of the three patients experienced complications at the 6-month follow up and all were doing well.
“The KONECT AVC represents an incremental step forward in aortic root replacement. In addition to saving time in the operating room, the KONECT AVC offers several potential advantages over traditional “back table” bio-Bentall grafts.”

FIGURE 1 KONECT RESILIA aortic valved conduit (Edwards Lifesciences, Irvine, CA).
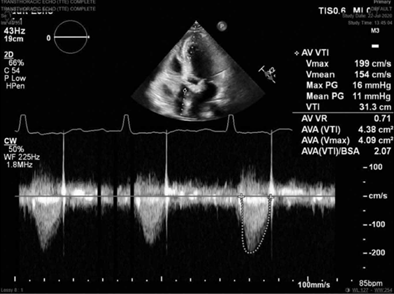
FIGURE 2 Representative predischarge transthoracic echocardiography image with continuous-wave Doppler demonstrating low postoperative aortic valve gradient and velocity in the KONECT RESILIA aortic valved conduit (Edwards Lifesciences, Irvine, CA).
The case studies in this paper demonstrate the process, benefits, and intra-operative outcomes of implantation of the KONECT RESILIA AVC, including how the conduit simplifies aortic root replacement. Long-term follow-up is necessary to determine whether there are additional advantages in terms of durability and hemodynamics relative to other commercially available bioprosthetic root replacement options.
To learn more about this product, visit Edwards.com/KONECT.
*Consult instructions for use for device preparation instructions.
†As compared to self-assembled tissue valved conduits.

