The ENCIRCLE Trial


About the trial

A prospective, multicenter, single-arm adaptive design study to evaluate the safety and effectiveness of transcatheter mitral valve replacement with the Edwards SAPIEN M3 system in patients with symptomatic, at least 3+ mitral regurgitation for whom commercially available surgical or transcatheter treatment options are deemed unsuitable.
Mitral Regurgitation Classification
Mitral regurgitation (MR) etiology can be categorized into primary or secondary. In primary MR (also knows as degenerative), there is an abnormality in one or more components of the mitral apparatus (leaflets, annulus, chordae tendineae, papillary muscles). In secondary MR (also known as functional), the valve itself is usually normal but mitral insufficiency arises from alterations in left ventricular (LV) geometry.

MR can also be described by the Carpentier classification*, which categorizes MR based on leaflet motion. There are four types – Type 1, Type 2, Type IIIa, and Type IIIb. Type 1 refers to valve dysfunction with normal leaflet motion, and includes annular dilation and leaflet perforation. Type II refers to mitral leaflets with increased mobility, so instances of prolapse or flail. Type IIIa refers to mitral leaflets that are restricted in both systole and diastole – including leaflets that are thickened and/or calcified due to rheumatic heart disease or stenosis. Type IIIb refers to mitral leaflets that are restricted only in systole, including LV wall motion abnormalities or left ventricular dilatation that result in chordal tethering.
* Carpentier A, Adams DH, and Filsoufi F. Carpentier's Reconstructive Valve Surgery. Saunders/Elsevier, 2010.


The ENCIRCLE trial is studying the SAPIEN M3 system in patients with symptomatic, at least 3+ MR of primary or secondary etiology and Carpentier functional classifications Type I, II, IIIa or IIIb mitral regurgitation. (All enrollment criteria must be met.)
The ENCIRCLE Trial
Purpose
This is a single-arm, multicenter study to establish the safety and effectivenes of the Edwards SAPIEN M3 transcatheter mitral valve replacement system in subjects with symptomatic, at least 3+ mitral regurgitation (MR) for whom commercially available surgical or transcatheter treatment options are deemed unsuitable.
Trial Design
The ENCIRCLE trial has a main study and two registries, enrolling subjects deemed unsuitable for commercially available surgical or transcatheter treatment options.†
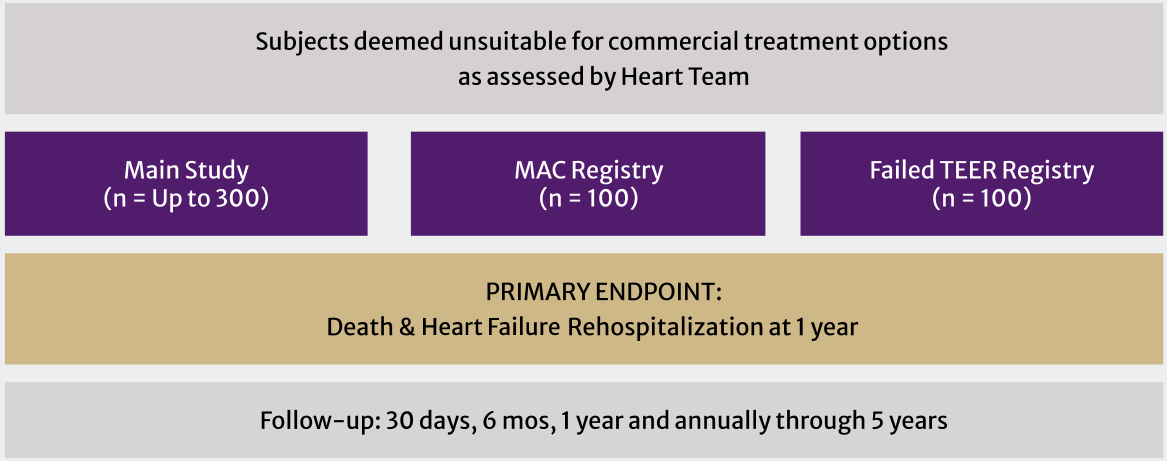
† Refer to Clinical Study Protocol for full enrollment criteria
Main Study
Subjects who meet the study criteria for whom commercially available surgical or transcatheter treatment options are deemed unsuitable will have transcatheter mitral valve replacement (TMVR)
Mitral Annular Calcification (MAC) Registry:
Up to 100 additional subjects with MAC will be treated in this Registry. Severe MAC is not required for entry into this Registry
Failed Transcatheter Edge-to-Edge Repair (TEER) Registry:
Subjects must be symptomatic, have at least 3+ mitral regurgitation (MR), and have had an attempted but failed TEER procedure
Eligibility and Enrollment Criteria§
- MR ≥ 3+ (alternate MAC Registry eligibility below)
- Refer to Clinical Study Protocol for alternate MAC Registry eligibility
- NYHA functional class ≥ II
- Commercially available surgical or transcatheter treatment options are deemed unsuitable due to clinical, anatomic, or technical considerations as determined by the Heart Team
- Optimized heart failure management based on subject characteristics and applicable guidelines, and stable for at least 30 days prior to enrollment (Failed TEER Registry exception)
§ Refer to Clinical Study Protocol for full inclusion and exclusion criteria
The Edwards SAPIEN M3 Transcatheter Mitral Valve Replacement System
The SAPIEN M3 system is a fully transseptal mitral valve replacement therapy.

The SAPIEN M3 dock:
- Nitinol dock designed to encircle native mitral leaflets to provide an anchor for the SAPIEN M3 valve
The SAPIEN M3 valve:
- Specifically engineered to respect the native mitral anatomy
- Leverages 29 mm SAPIEN 3 valve tissue and frame
- Includes full-frame PET skirt
Watch the SAPIEN M3 system procedural animation

We're here for you
We are committed to providing the highest levels of customer service to help our patients improve their quality of life. For any questions, or if you are interested in being part of this study, contact Edwards Patient Support Center. For details about the trial, visit NCT04153292 at ClinicalTrials.gov.
Give us a call
Send us an email
