Transcatheter mitral repair clinical trials


About this trial
A prospective, multicenter, randomized, controlled pivotal trial to evaluate the safety and effectiveness of transcatheter mitral valve repair with the Edwards PASCAL Transcatheter Valve Repair System compared with Abbott MitraClip in patients with degenerative mitral regurgitation.1 This trial has completed enrollment.

Degenerative mitral regurgitation (DMR)
Degenerative mitral regurgitation (also known as Primary MR) is caused by an intrinsic abnormality to the mitral valve apparatus (leaflets, chordae, papillary muscles, and annulus). The abnormalities of the valve apparatus cause systolic regurgitation of blood from the left ventricle to the left atrium, leading to volume overload.3,4
The Edwards PASCAL transcatheter valve repair system
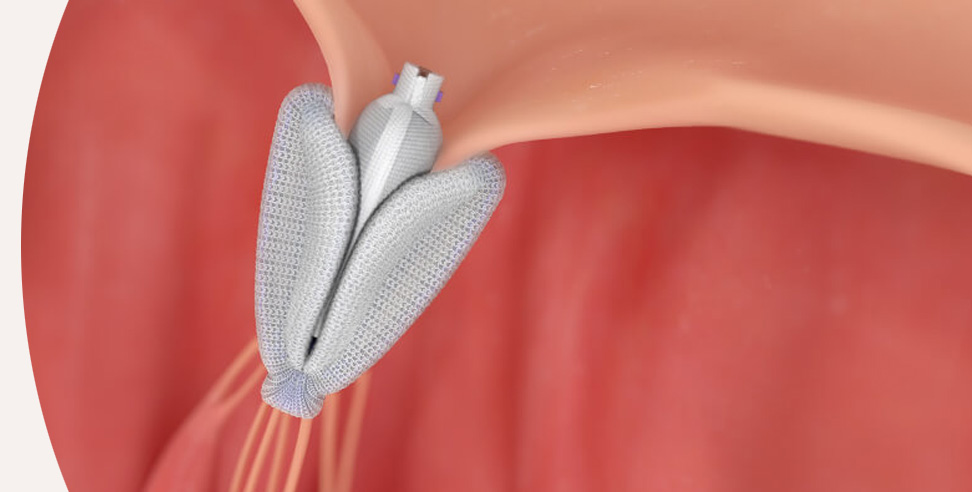
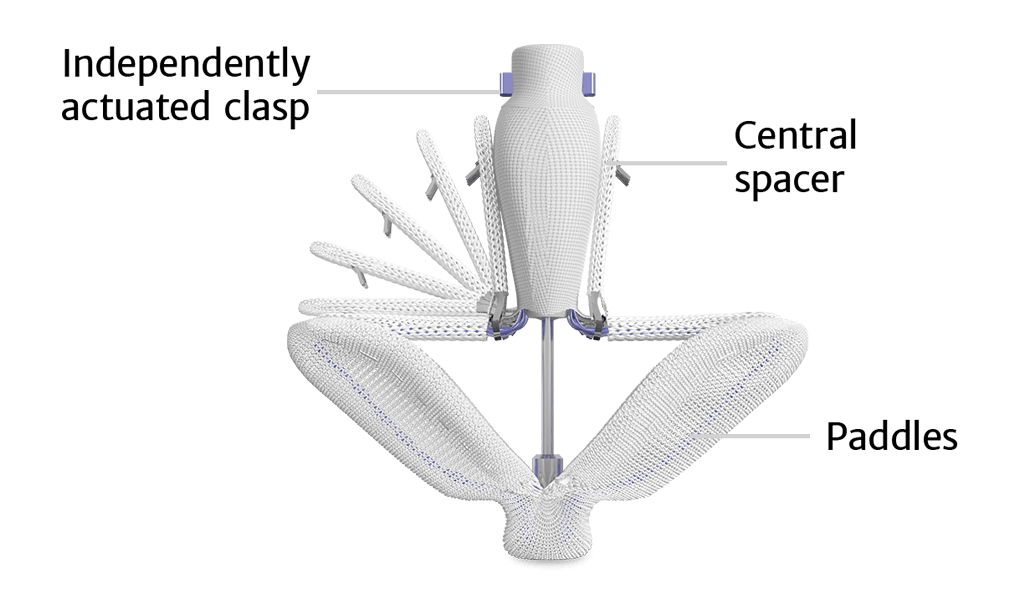
The PASCAL implant
- Central spacer intended to fill the regurgitant orifice area
- Spacer and broad, contoured paddle design reduce stress on leaflets
- Clasps allow for independent leaflet capture and the ability to fine-tune leaflet position
See how it works
CLASP IID trial design
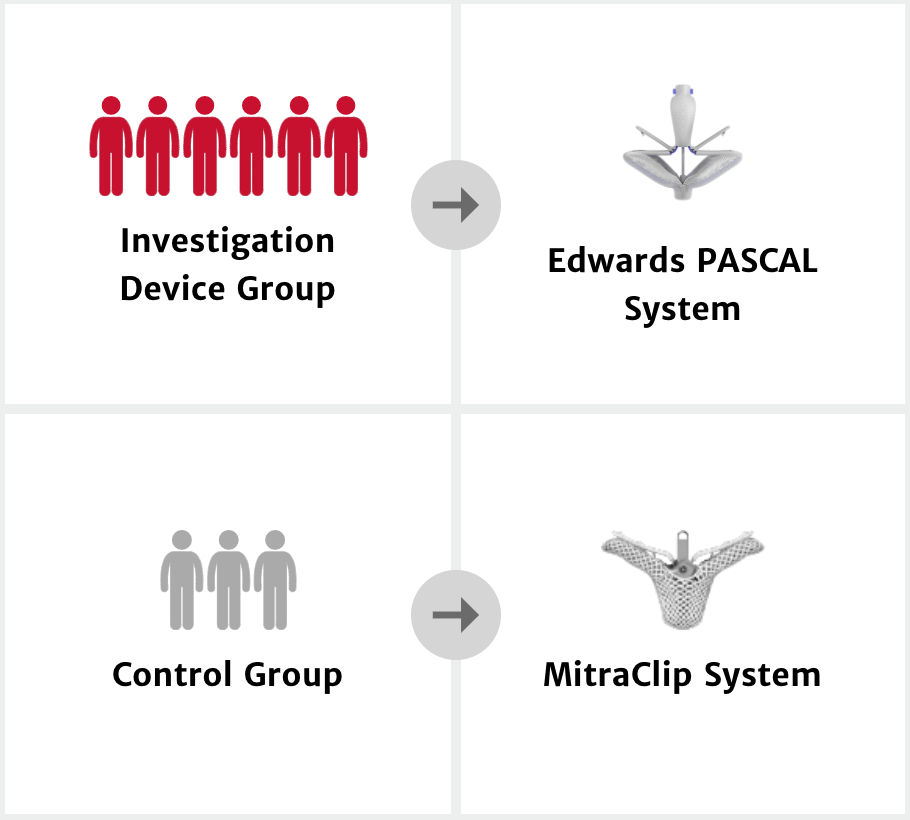
The CLASP IID cohort purpose1
The CLASP IID cohort design
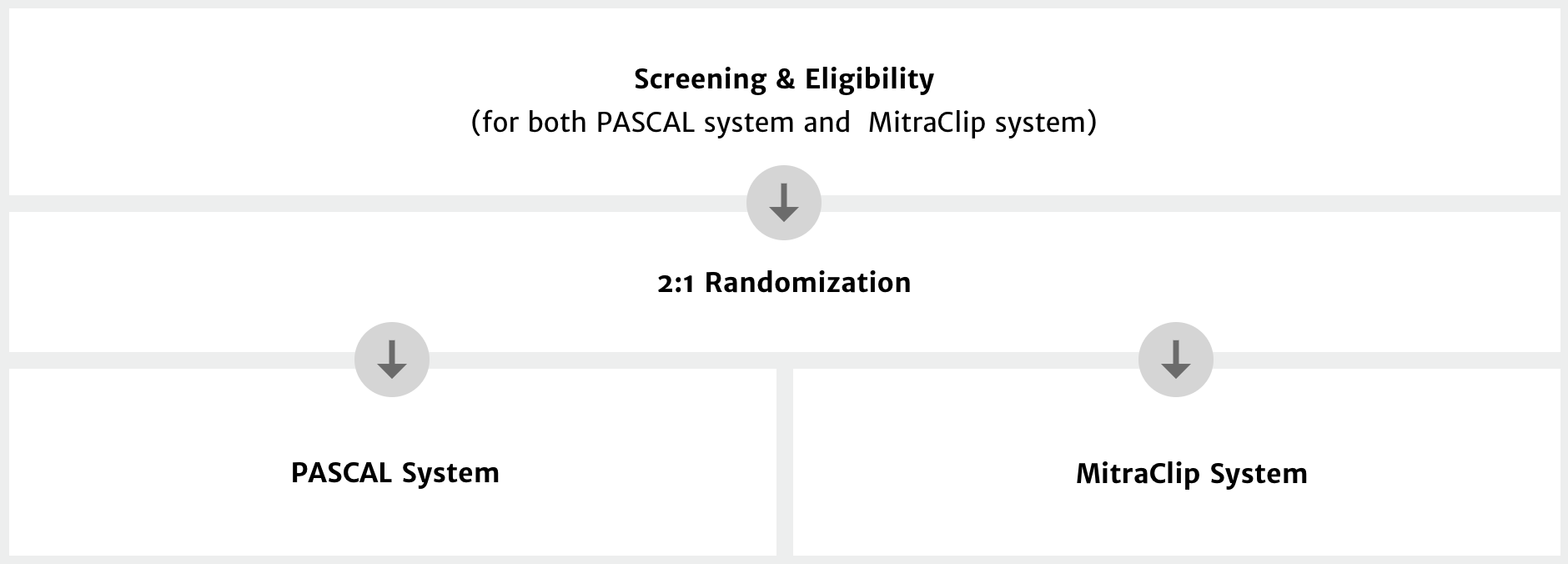
Eligibility and enrollment criteria
Key inclusion criteria1
- Patient has 3+ or 4+ mitral regurgitation, as determined by echo
- Patient is determined by the Heart Team to be a candidate for transcatheter mitral valve repair for both PASCAL and MitraClip
- Patient is determined to be at prohibitive risk for mitral valve surgery by a Heart Team
Key exclusion criteria1
- Mitral valve anatomy which may preclude proper PASCAL or MitraClip access, use and/or deployment, or sufficient reduction in mitral regurgitation
- Patient with refractory heart failure requiring advanced intervention (ie, left ventricular assist device, transplantation, ACC/AHA Stage D heart failure)
- Any prior mitral valve surgery or transcatheter mitral valve procedure (excluding chordal replacement or surgical annuloplasty repair)
- Other severe valve disorders requiring intervention
- Need for emergent or urgent surgery for any reason or any planned cardiac surgery within the next 12 months
Patient selection
screening & enrollment


The Heart Team approach for the CLASP IID cohort

Patient screening process
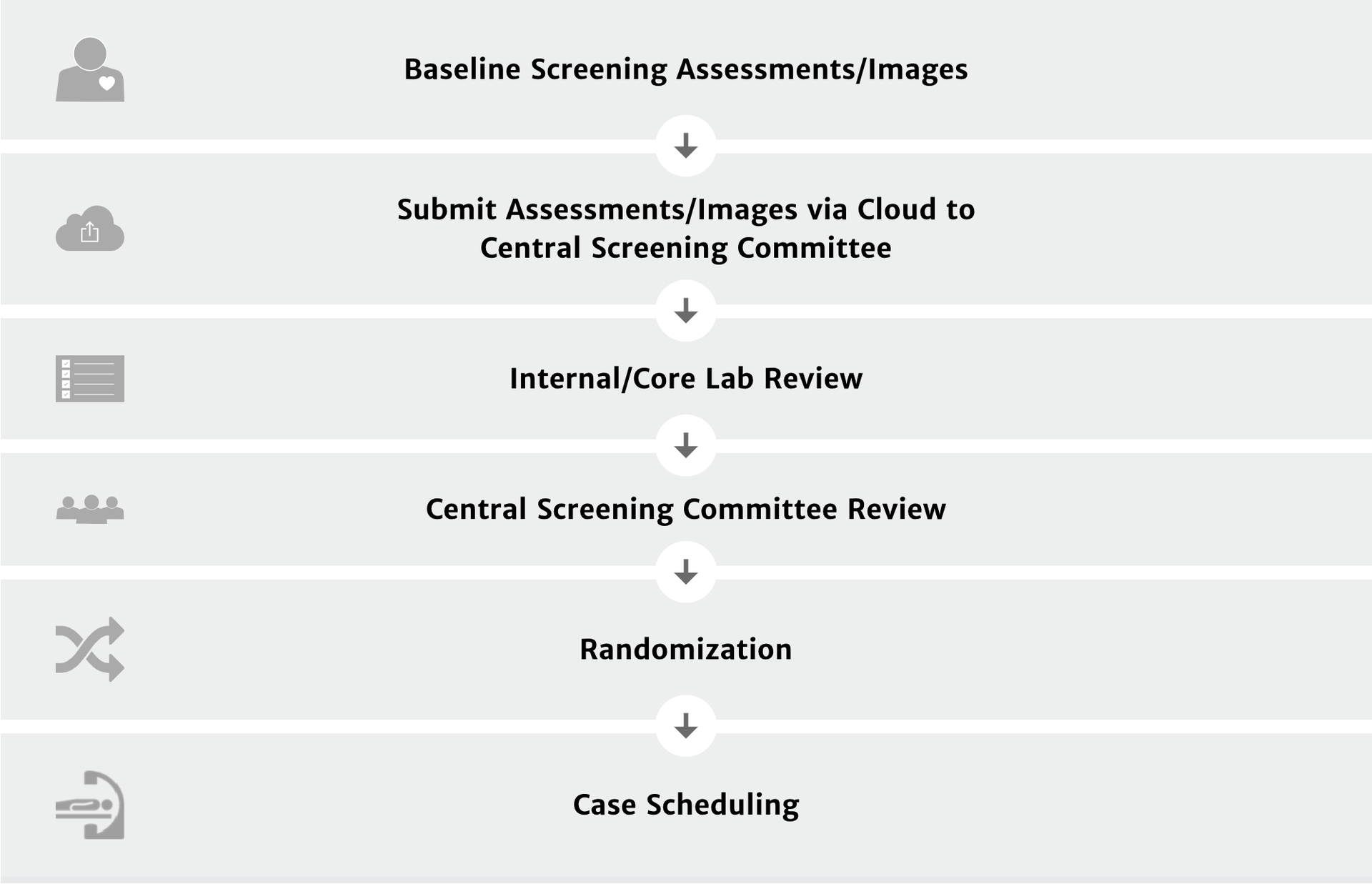
Patient follow up1:


We're here for you
We are committed to providing the highest levels of customer service to help our patients improve their quality of life. For details about the trial, visit NCT03706833 at Clinical Trials.gov.
References
- Edwards PASCAL CLASP IID/IIF Pivotal Clinical Trial.
- Mitral Valve Regurgitation: Symptoms and Causes. Mayo Clinic: Mayo Clinic website. https://www.mayoclinic.org/diseases-conditions/mitral-valve-regurgitation/symptoms-causes/syc-20350178. Accessed May 21, 2020.
- Apostolidou E, Maslow AD, Poppas A. Primary mitral valve regurgitation: Update and review. Glob Cardiol Sci Pract. 2017;2017(1):e201703.
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:e57-e185.


