Key inclusion criteria
- LVEF >40%
- NYHA Class II-IVa
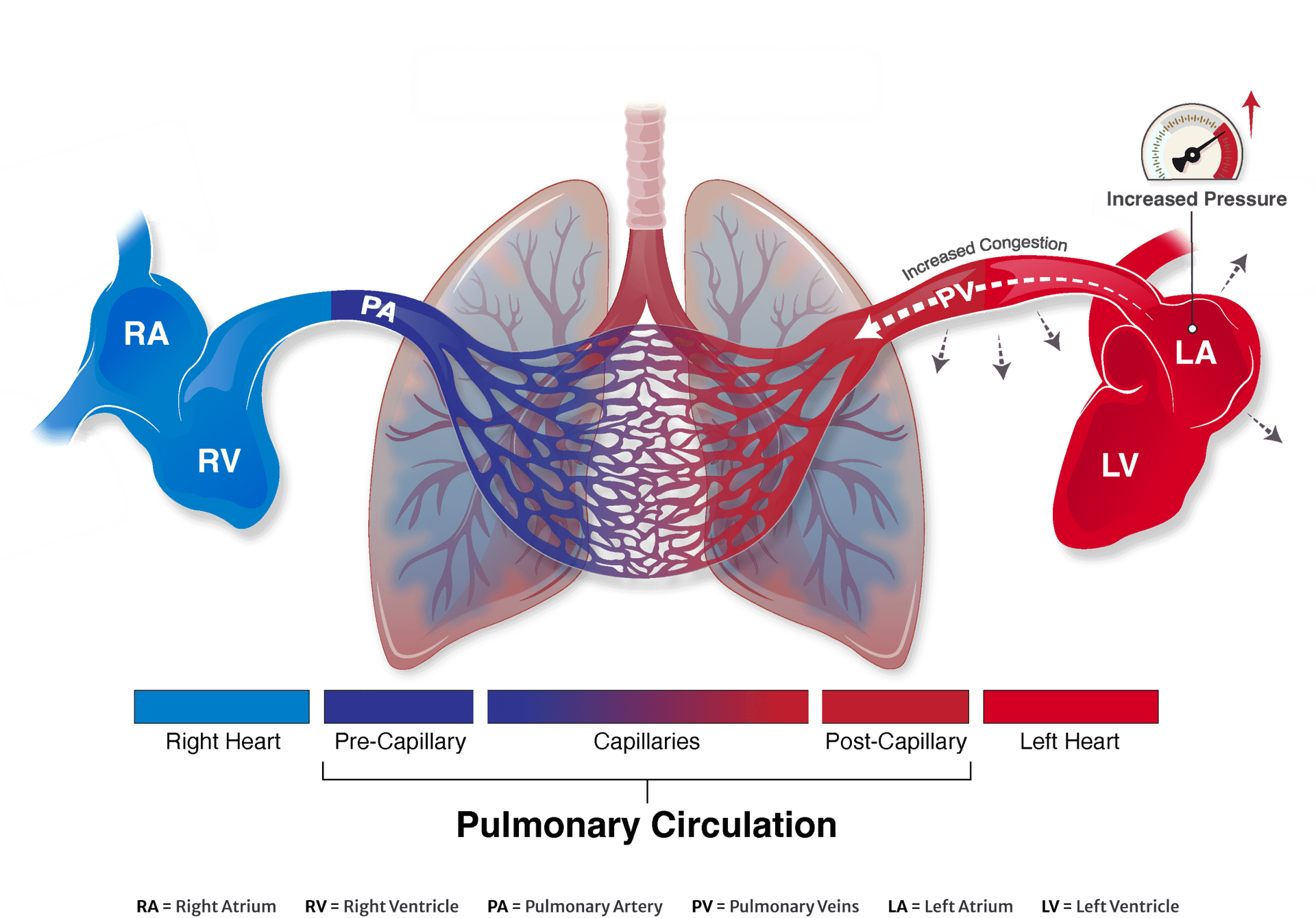
- HF hospitalization or HF event requiring IV therapy or intensification of oral diuretics in the prior 12 months OR elevated BNP or NT-proBNP in the past 6 months
- PCWP at ≥ 20W exercise is ≥ 25mmHg AND exceeds RAP by at least 8mmHg
- Stable GDMT for at least 30 days prior to screening assessments