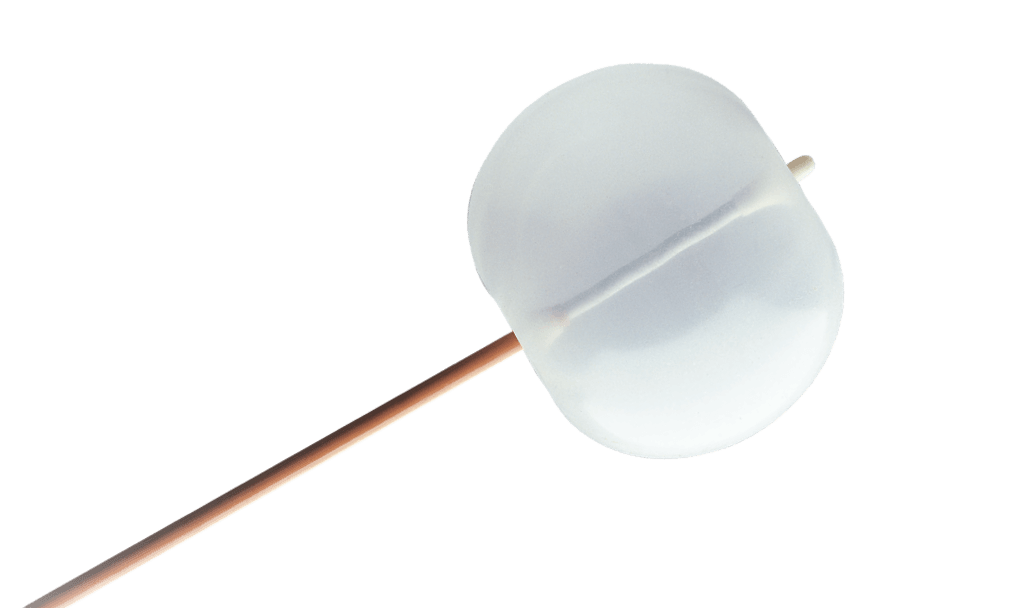
Vascushunt II silicone carotid shunt

Edwards Lifesciences Critical Care product group is now part of BD (Becton, Dickinson and Company). Edwards is the manufacturer of the products.
To find the latest information, please visit this page on BD.com
Vascushunt II silicone carotid endarterectomy shunt
Flexibility in shunting … All the capabilities of Vascushunt carotid shunt, with a safety balloon and choice of sizes.
Safety balloon
- The incorporation of a safety balloon is designed to reduce the possibility of damaging the internal carotid artery.
- If the internal carotid balloon inadvertently overinflates, excess pressure is diverted to the safety balloon, reducing pressure in the internal balloon to help minimize the possibility of vessel trauma.*
Flexibility
- The Vascushunt II silicone carotid endarterectomy shunt is designed to provide flexibility for ease-of-use during the procedure.
Multiple sizes and lengths
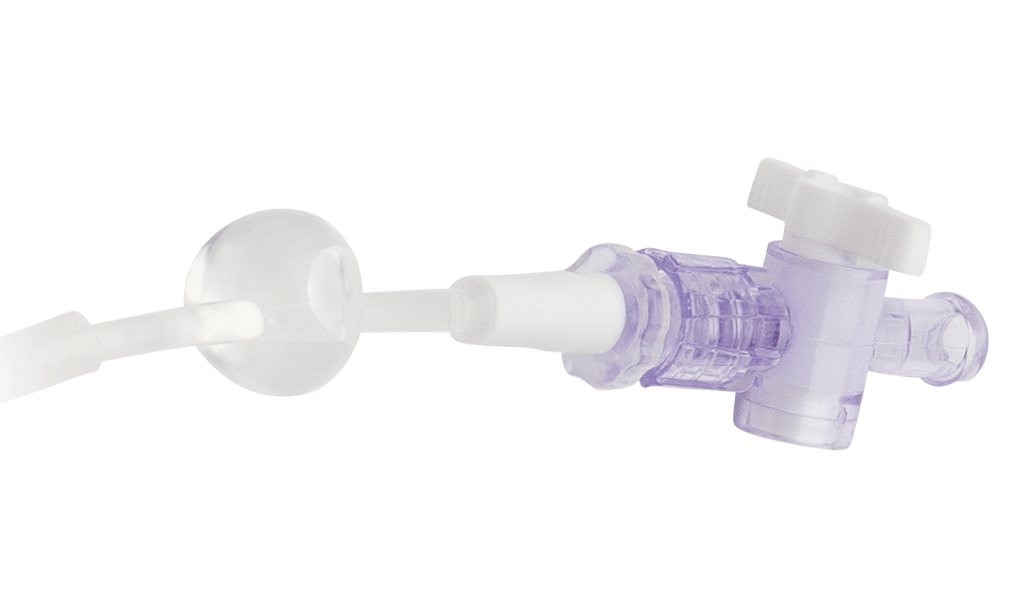
- Available in 8F, 9F and 11F in both outlying 30 cm and inlying 15 cm lengths, with stopcocks to meet a variety of clinical needs.
Caution: Care must be taken at all times to prevent over-inflation of the balloons, as it may result in obstruction of blood flow and injury to the vessel.
Vascushunt II silicone endarterectomy shunt is not made with natural rubber latex.
Manufactured by Lucas Medical, Inc. Distributed by Edwards Lifesciences LLC.
*It has been reported that human internal carotid arteries can rupture at pressures of 20 psi. The safety balloon is intended to prevent the pressure from reaching this level by inflating at a level that is less than 20 psi.
Models
Vascushunt II silicone balloon shunts
| Model Number | French Size | Length |
| T38F15 | 8F | 15 cm (Inlying) |
| T38F30 | 8F | 30 cm (Outlying) |
| T39F15 | 9F | 15 cm (Inlying) |
| T39F30 | 9F | 30 cm (Outlying) |
| T311F15 | 11F | 15 cm (Inlying) |
| T311F30 | 11F | 30 cm (Outlying) |
Related products
Important safety information
CAUTION: Federal (United States) law restricts this device to sale by or on the order of a physician.
See Instructions For Use (IFU) / Directions For Use (DFU) for full prescribing information, including indications, contraindications, warnings, precautions and adverse events.