
people in the United States are estimated to have clinically relevant TR5,6

Medications may treat symptoms but not the TR itself, which can continue to progress. Reducing TR severity may improve patient quality of life.2,4

people in the United States are estimated to have clinically relevant TR5,6

of patients with mild or trivial TR progressed to moderate or severe TR in ~3 years§,7

estimated mortality in patients with severe TR within 1 year of diagnosis8, 9
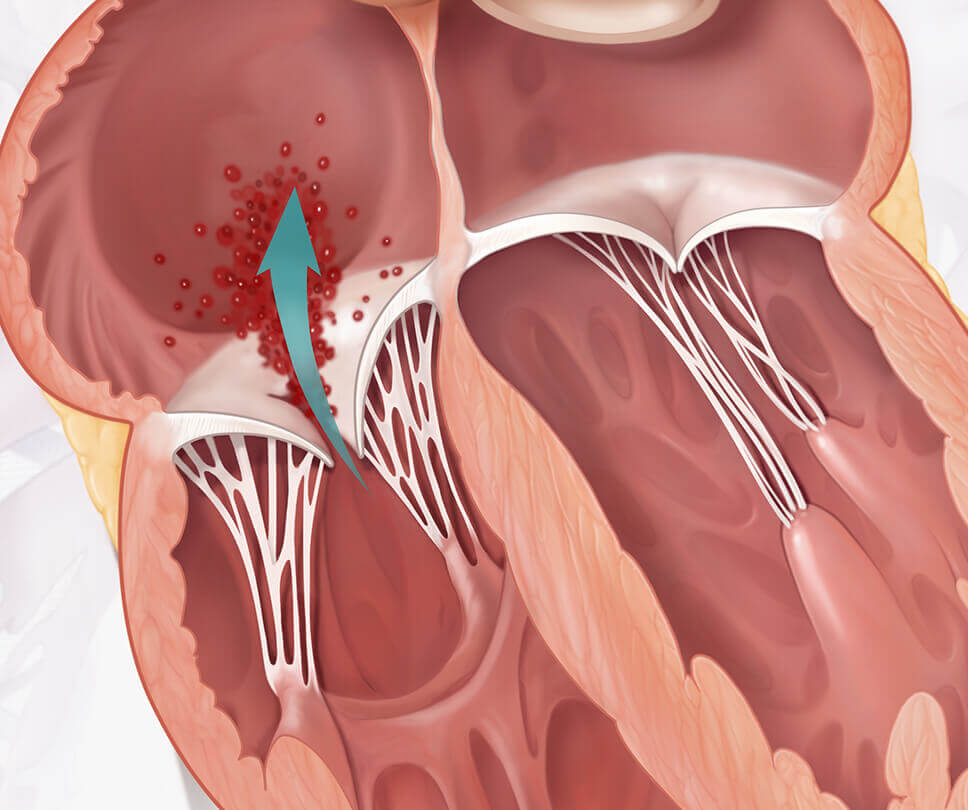
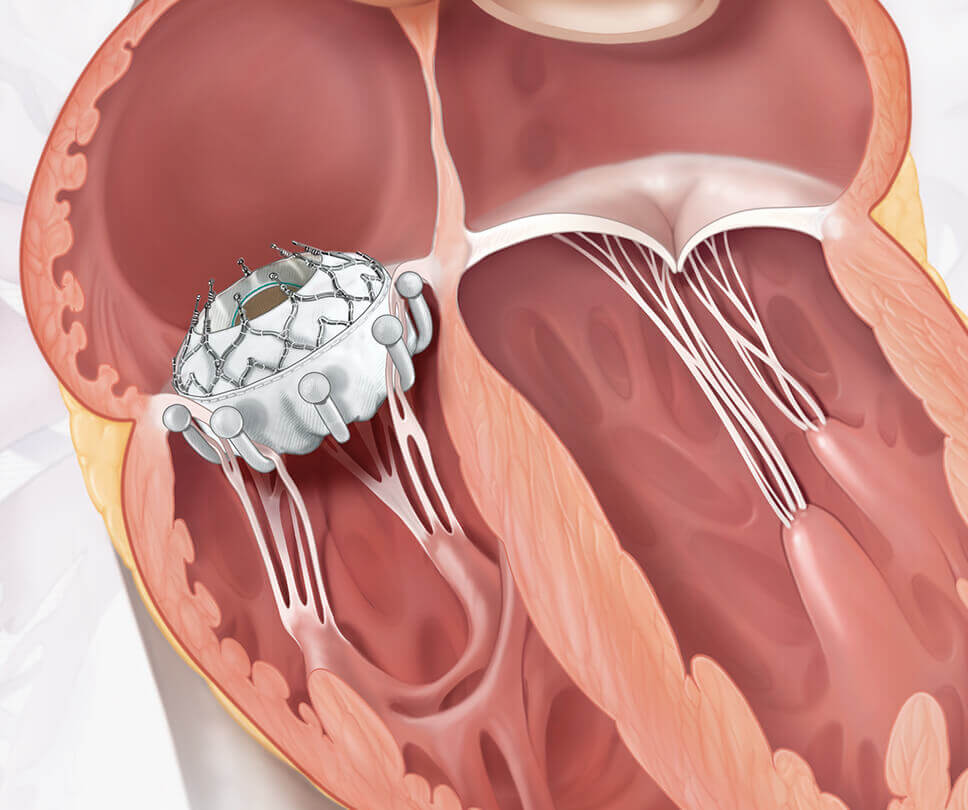
Move the arrow left or right to see the comparison between a heart with tricuspid regurgitation and one with the EVOQUE valve


Reducing TR severity may improve patient quality of life2,4
§Based on a retrospective echocardiographic analysis of Israeli patients (n=1,552).
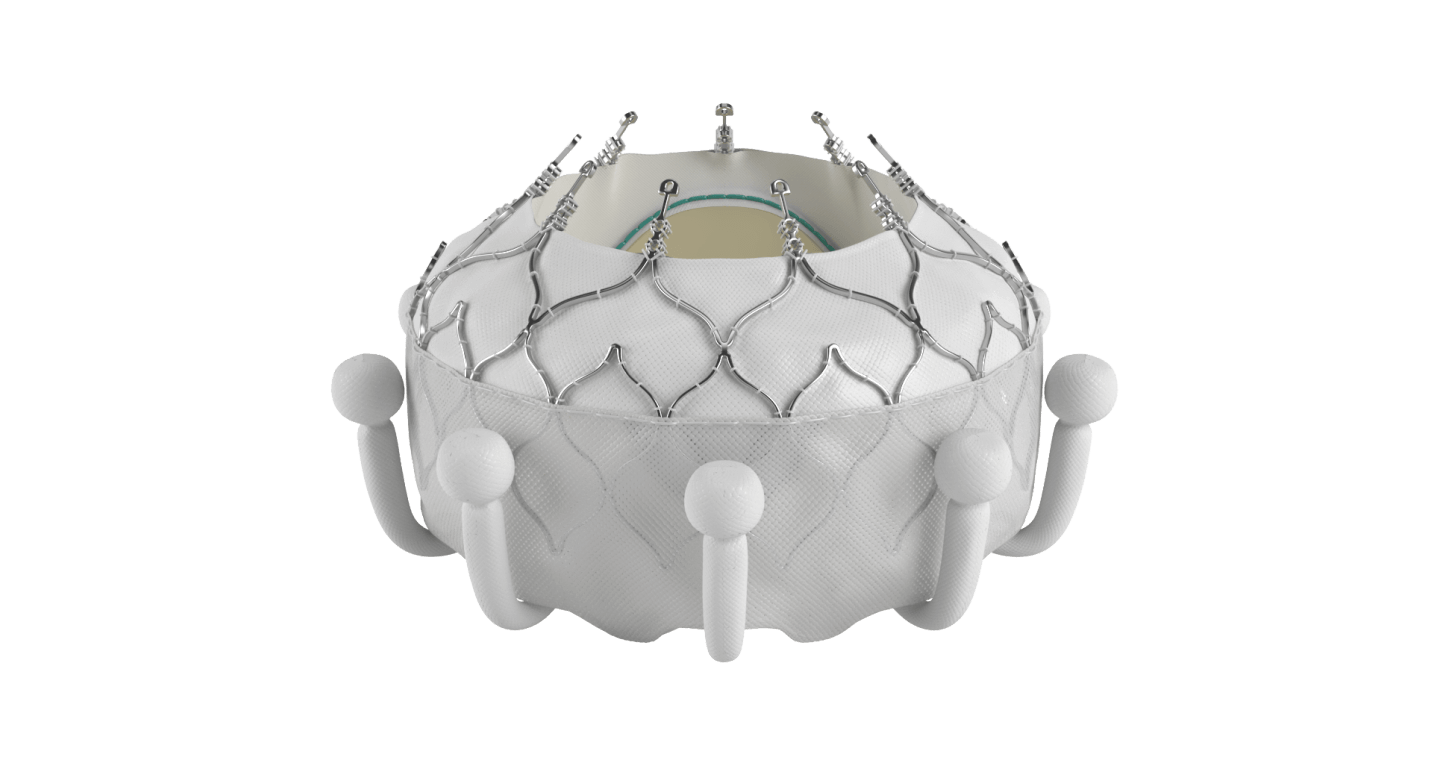
The EVOQUE system is the world's first transcatheter tricuspid valve replacement therapy
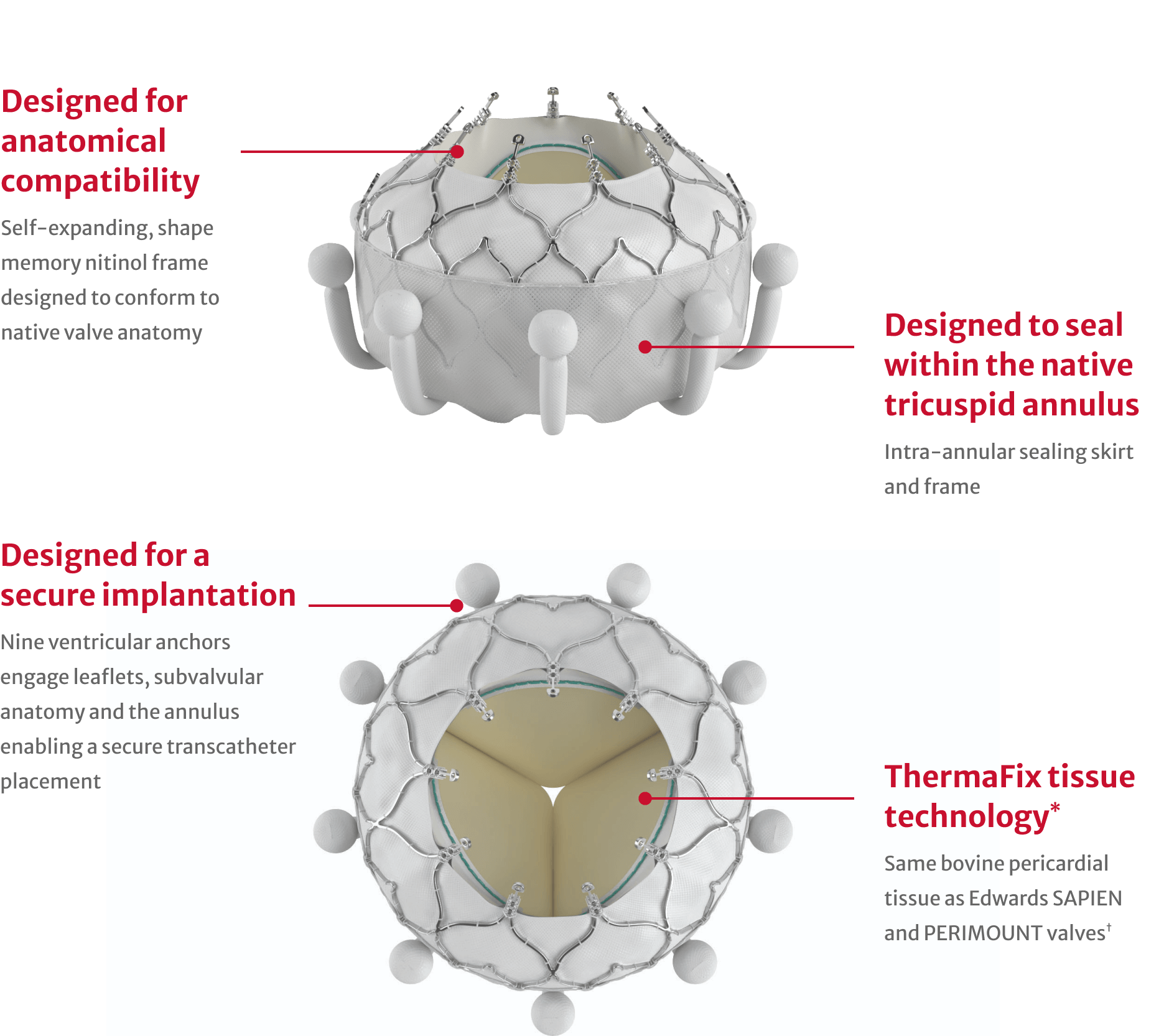
*No clinical data are available that evaluates long-term impact of the Carpentier-Edwards ThermaFix tissue process in patients.
†Excluding Edwards SAPIEN 3 Ultra RESILIA valves.

Procedural efficiency with an average device time of approximately one hour
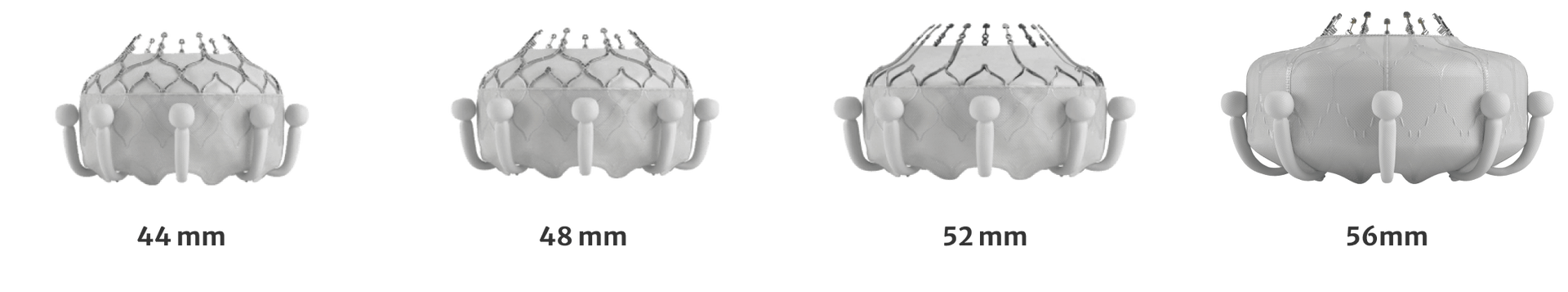
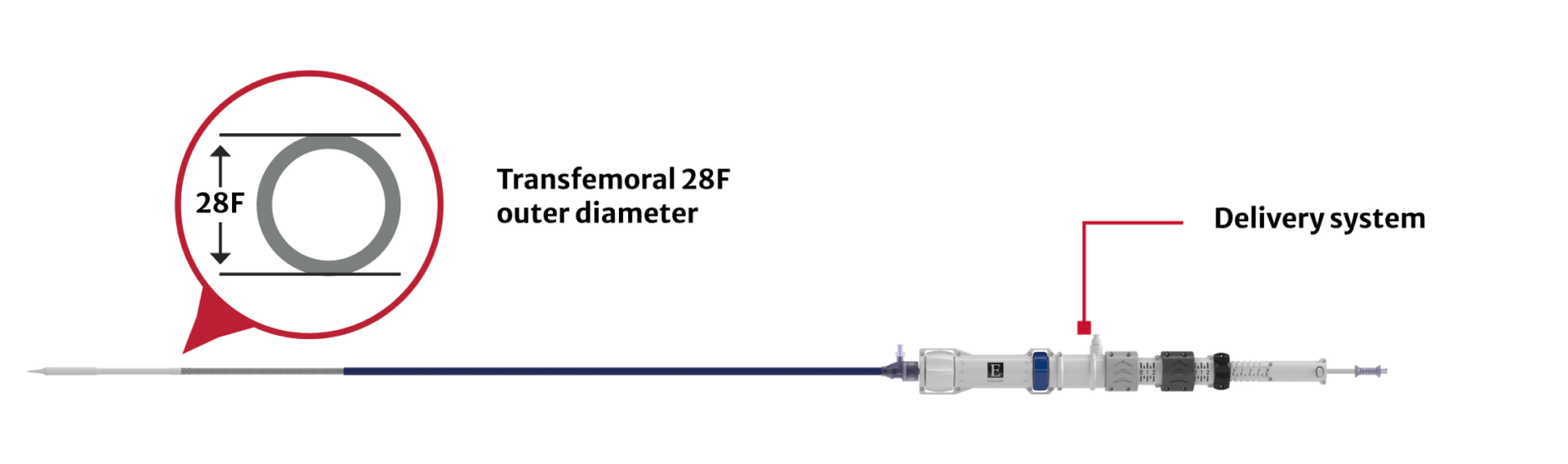
Same delivery system for all valve sizes
Superior clinical benefit and consistent TR resolution for patients with EVOQUE TTVR

The TRISCEND II trial is a prospective multi-center randomized controlled trial evaluating the safety and effectiveness of transcatheter tricuspid valve replacement with the EVOQUE system plus optimal medical therapy compared to optimal medical therapy alone in patients with symptomatic severe TR. These data pertain to the entire cohort of 400 patients at 1 year.
The TRISCEND II trial successfully met the composite primary safety and effectiveness endpoint at 1 year.
Greater likelihood of clinical benefit with EVOQUE TTVR vs medical therapy alone
(95% CI, 1.56 to 2.62; p<0.001)

Greater likelihood of clinical benefit with EVOQUE TTVR vs medical therapy alone
(95% CI, 1.56 to 2.62; p<0.001)


Composite Endpoint

Win Ratio Analysis
Greater likelihood of clinical benefit with EVOQUE TTVR vs medical therapy alone
(95% CI, 1.56 to 2.62; p<0.001)
Greater likelihood of clinical benefit with EVOQUE TTVR vs medical therapy alone
(95% CI, 1.56 to 2.62; p<0.001)
≤mild TR with EVOQUE TTVR at 1 year
median device time




TR Reduction

Treats a diverse patient population

Treats a broad patient population
≤mild TR with EVOQUE TTVR at 1 year
median device time
Favorable trends observed in all-cause mortality and heart failure hospitalizations at 1 year between EVOQUE TTVR and medical therapy alone.
with EVOQUE TTVR vs 15.2% with medical therapy alone

with EVOQUE TTVR vs 26.1% with medical therapy alone

Cardiovascular mortality
Stroke
Severe bleeding
New pacemaker implant in pacemaker-naïve patients


Kaplan-Meier All-Cause Mortality

Kaplan-Meier Heart Failure Hospitalization

30-day Safety profile
with EVOQUE TTVR vs 15.2% with medical therapy alone
with EVOQUE TTVR vs 26.1% with medical therapy alone
Cardiovascular mortality
Stroke
Severe bleeding
New pacemaker implant in pacemaker-naïve patients
At 1-year follow-up compared to baseline, patients treated with EVOQUE TTVR experienced meaningful and sustained TR improvements in quality-of-life, functional status, and exercise capacity.
improvement in KCCQ-OS with EVOQUE TTVR at 1 year compared to baseline

of EVOQUE TTVR patients in NYHA Class I/II at 1 year

improvement in 6MWD with EVOQUE TTVR at 1 year compared to baseline


KCCQ-OS improvement

NYHA Class improvement

6MWD improvement
improvement in KCCQ-OS with EVOQUE TTVR at 1 year compared to baseline
of EVOQUE TTVR patients in NYHA Class I/II at 1 year
improvement in 6MWD with EVOQUE TTVR at 1 year compared to baseline

Indications: The EVOQUE tricuspid valve replacement system is indicated for the improvement of health status in patients with symptomatic severe tricuspid regurgitation despite being treated optimally with medical therapy for whom tricuspid valve replacement is deemed appropriate by a Heart Team.
Contraindications: The EVOQUE valve is contraindicated in patients who cannot tolerate an anticoagulation/antiplatelet regimen, who have active bacterial endocarditis or other active infections, or who have untreatable hypersensitivity to nitinol alloys (nickel and titanium).
Warnings: The EVOQUE valve, delivery system, loading system, dilator kit, are designed, intended, and distributed as STERILE and for single use only. The positioning accessories are available in single use, nonsterile, disposable as well as reusable configurations, please refer to the device information and ensure the device is used as intended. Do not resterilize or reuse any of the single use devices. There are no data to support the sterility, nonpyrogenicity, or functionality of the single use devices after reprocessing. Ensure the correct valve size is selected. Implantation of the improper size (i.e., undersizing or oversizing) may lead to paravalvular leak (PVL), migration, embolization, and/or annular damage.
Patients with previously-implanted devices (e.g., IVC filter) should be carefully assessed prior to insertion of the delivery system to avoid potential damage to vasculature or a previously-implanted device. Patients with pre-existing cardiac leads should be carefully assessed prior to implantation to avoid potential adverse interaction between devices. Care should be taken when implanting cardiac leads after EVOQUE valve implantation to avoid potential adverse interaction between the devices. Patients implanted with the EVOQUE valve should be maintained on anticoagulant/antiplatelet therapy as determined by their physicians in accordance with current guidelines, to minimize the risk of valve thrombosis or thromboembolic events.
There are no data to support device safety and performance if the patient has: echocardiographic evidence of severe right ventricular dysfunction; pulmonary arterial systolic pressure (PASP) > 70 mmHg by echo Doppler; a trans-tricuspid pacemaker or defibrillator lead that has been implanted in the RV within the last 3 months; or dependency on a trans-tricuspid pacemaker without alternative pacing options.
Precautions: Prior to use, the patient’s eligibility depends on the anatomic conditions based on CT scan. It is advised that a multi-disciplinary heart team be of the opinion that EVOQUE valve implantation is preferable to alternative percutaneous device solutions, including minimally-invasive open heart surgery. It is advised that a multi-disciplinary heart team takes into consideration the severity of disease and the chances of reversibility of right heart failure based on a complete hemodynamic assessment.
The EVOQUE valve is to be used only with the EVOQUE delivery system and EVOQUE loading system. The procedure should be conducted under appropriate imaging modalities, such as transesophageal echocardiography (TEE), fluoroscopy, and/or intracardiac echocardiography (ICE). Glutaraldehyde may cause irritation of the skin, eyes, nose, and throat. Avoid prolonged or repeated exposure to, or breathing of, the solution. Use only with adequate ventilation. If skin contact occurs, immediately flush the affected area with water; in the event of contact with eyes, seek immediate medical attention. For more information about glutaraldehyde exposure, refer to the Safety Data Sheet available from Edwards Lifesciences. Conduction disturbances may occur before, during, or following implantation of the EVOQUE valve, which may require continuous ECG monitoring before hospital discharge. The risk of conduction disturbances may increase with the 56mm valve size. If a patient has confirmed or suspected conduction disturbances, consider patient monitoring and/or electrophysiology evaluation. Appropriate antibiotic prophylaxis is recommended post-procedure in patients at risk for prosthetic valve infection and endocarditis. Long-term durability has not been established for the EVOQUE valve. Regular medical follow-up is advised to evaluate EVOQUE valve performance. Implantation of the EVOQUE valve should be postponed in patients with (1) a history of myocardial infarction within one month (30 days) of planned intervention, (2) pulmonary emboli within 3 months (90 days) of planned intervention, (3) cerebrovascular accident (stroke or TIA) within 3 months (90 days) of planned intervention, (4) active upper GI bleeding within 3 months (90 days) prior to procedure requiring transfusion.
Potential Adverse Events: Potential adverse events related to standard cardiac catheterization, use of anesthesia, the EVOQUE valve, and the implantation procedure include: death; abnormal lab values; allergic reaction to anesthesia, contrast media, anti-coagulation medication, or device materials; anaphylactic shock; anemia or decreased hemoglobin (Hgb), may require transfusion; aneurysm or pseudoaneurysm; angina or chest pain; arrhythmia – atrial (i.e., atrial fibrillation, supraventricular tachycardia); arrhythmias – ventricular (i.e., ventricular tachycardia, ventricular fibrillation); arterio-venous fistula; bleeding; cardiac arrest; cardiac (heart) failure; cardiac injury, including perforation; cardiac tamponade / pericardial effusion; cardiogenic shock; chordal entanglement or rupture that may require intervention; coagulopathy, coagulation disorder, bleeding diathesis; conduction system injury, which may require implantation of a pacemaker (temporary or permanent); conversion to open heart surgery; coronary artery occlusion; damage to or interference with function of pacemaker or implantable cardioverter defibrillator (ICD); edema; electrolyte imbalance; embolization including air, particulate, calcific material, or thrombus; emergent cardiac surgery; endocarditis; esophageal irritation; esophageal perforation or stricture; EVOQUE system component(s) embolization; failure to retrieve any EVOQUE system components; fever; gastrointestinal bleeding; hematoma; hemodynamic compromise; hemolysis / hemolytic anemia; hemorrhage requiring transfusion/surgery; hypertension; hypotension; inflammation; injury to the tricuspid apparatus including chordal damage, rupture, papillary muscle damage; local and systemic infection; mesenteric ischemia or bowel infarction; multi-system organ failure; myocardial infarction; nausea and/or vomiting; nerve injury; neurological symptoms, including dyskinesia, without diagnosis of TIA or stroke; non-emergent reoperation; pain; pannus formation; paralysis; percutaneous valve intervention; peripheral ischemia; permanent disability; pleural effusion; pneumonia; pulmonary edema; pulmonary embolism; reaction to anti-platelet or anticoagulation agents; rehospitalization; renal failure; respiratory failure, atelectasis – may require prolonged intubation; retroperitoneal bleed; right ventricular outflow tract (RVOT) obstruction; septicemia, sepsis; skin burn, injury, or tissue changes due to exposure to ionizing radiation; stroke; structural deterioration (wear, fracture, calcification, leaflet tear, leaflet thickening, stenosis of implanted device, or new leaflet motion disorder); thromboembolism; transient ischemic attack (TIA); valve dislodgement/embolization; valve endocarditis; valve explant; valve leaflet entrapment; valve malposition; valve migration; valve paravalvular leak (PVL); valve regurgitation (new or worsening tricuspid, aortic, mitral, pulmonary); valve thrombosis; vascular injury or trauma, including dissection or occlusion; vessel spasm ; wound dehiscence, delayed or incomplete healing.
CAUTION: Federal (United States) law restricts this device to sale by or on the order of a physician. See instructions for use for full prescribing information.
For more information, see Edwards.com/EVOQUE
Receive event and news information from Edwards Lifesciences