PASCAL implant
Designed to reduce leaflet stress and increase open orifice area enabling low gradients
Feb 22 is Heart Valve Disease Awareness Day — Learn more about the HVD symptoms at Listen to your Heart
For degenerative mitral regurgitation
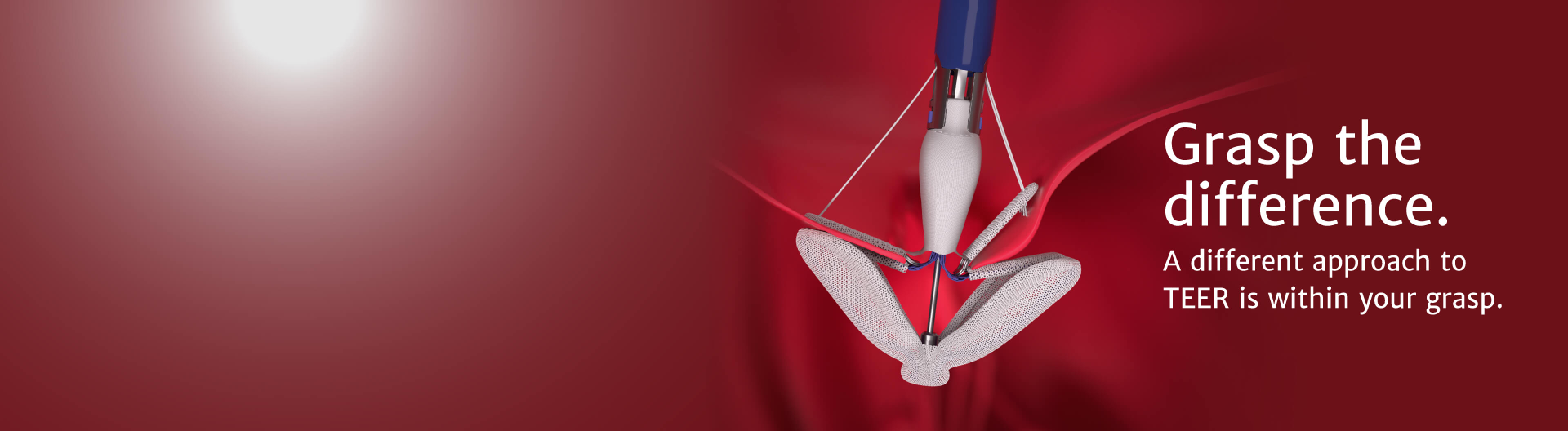
The latest advancement from Edwards Lifesciences in transcatheter mitral therapies: The PASCAL Precision system
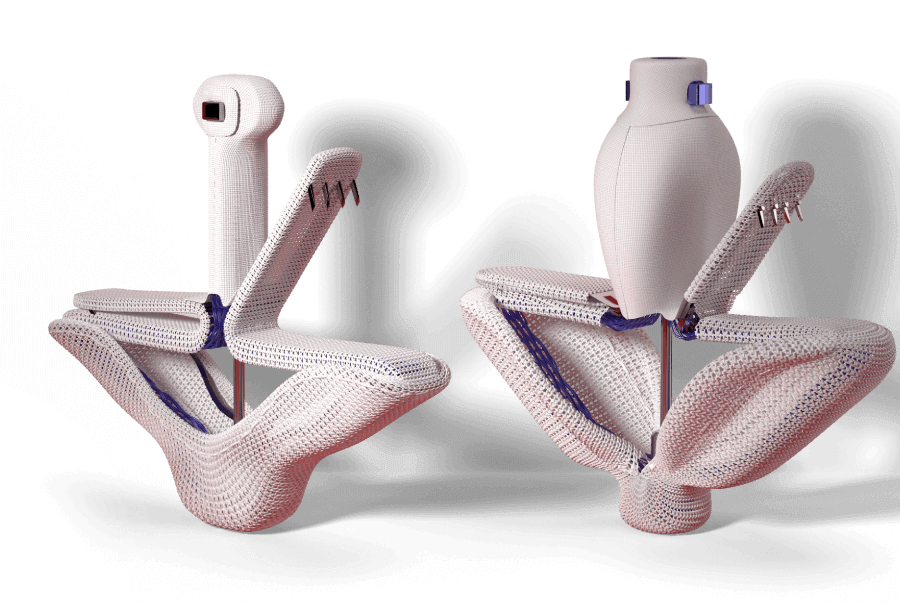
Designed to reduce leaflet stress and increase open orifice area enabling low gradients
Features a narrower design profile to help you optimize your treatment of degenerative mitral regurgitation

Treat your patients with degenerative mitral regurgitation with the latest transcatheter mitral innovation from Edwards Lifesciences
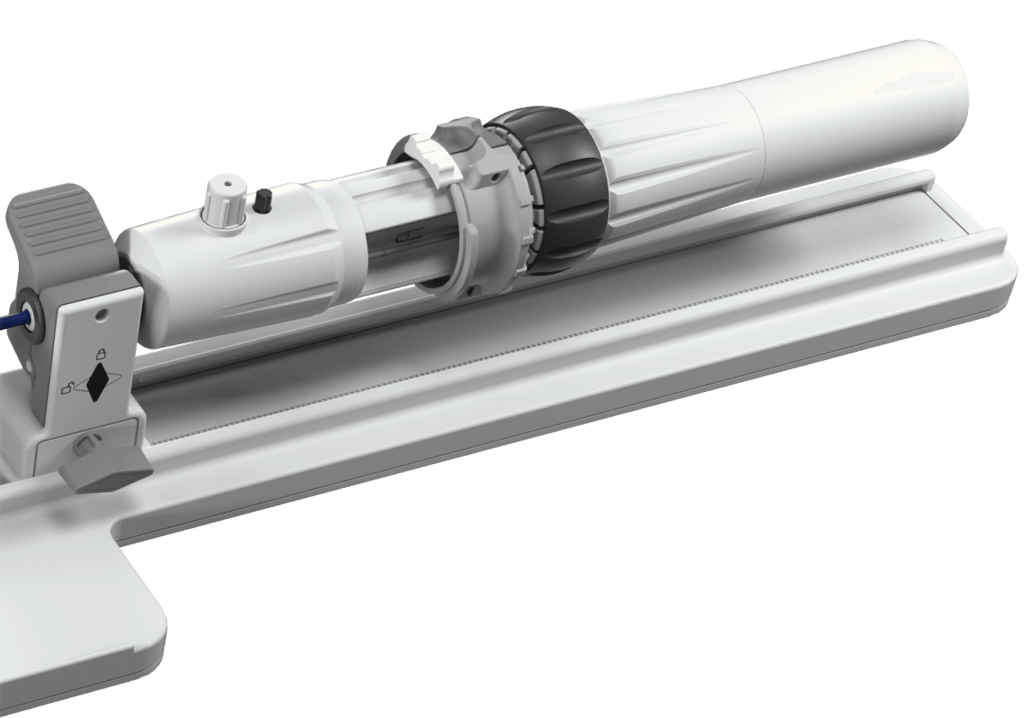
Adapt to specific procedural and anatomical needs
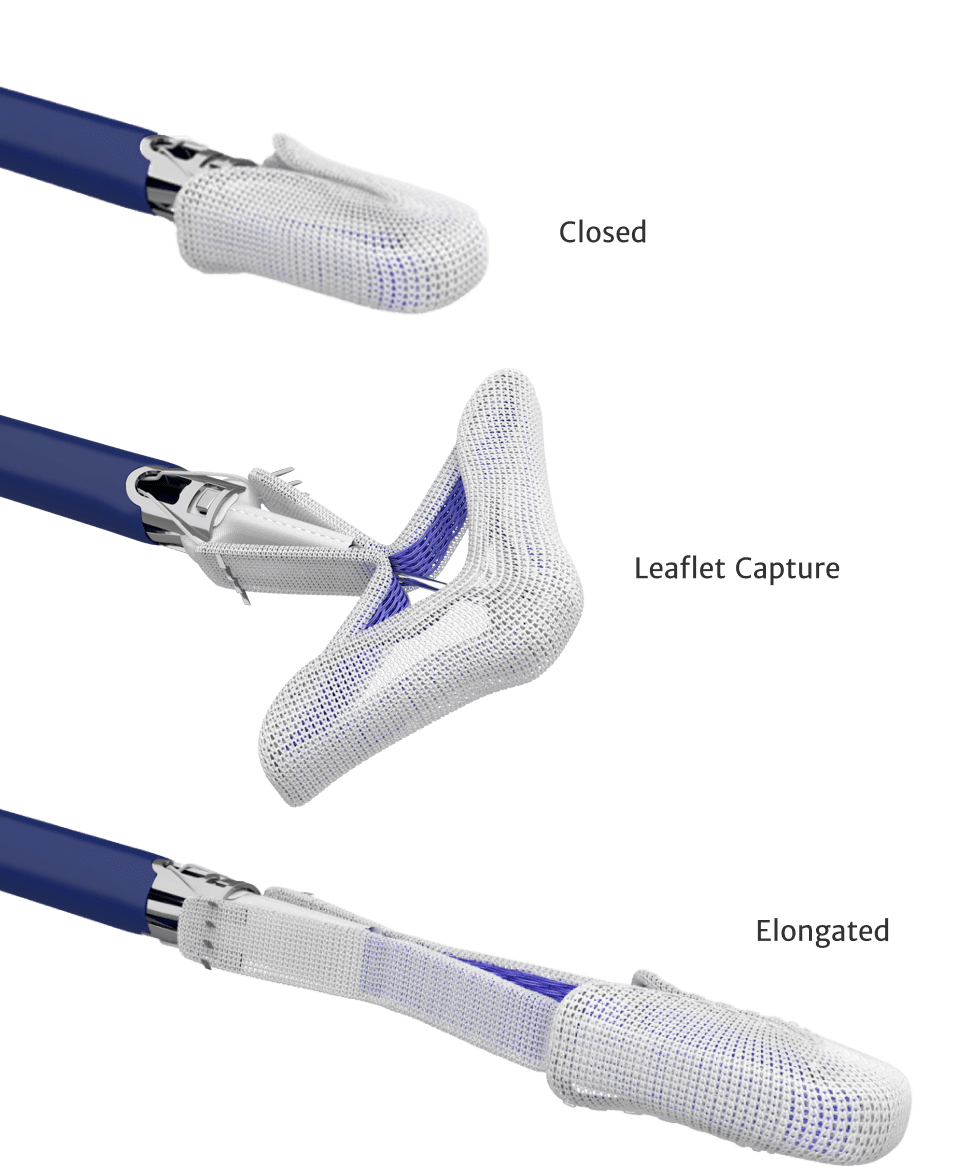

CLASP IID Randomized Control Trial (RCT): The first and only RCT to directly compare two contemporary TEER therapy in patients with DMR.
CLASP IID Registry: A first-of-its-kind registry to evaluate the PASCAL system in DMR patients with complex anatomy.
CLASP: The first international, multicenter study to evaluate the safety and clinical outcomes of the PASCAL system.

The objective of this prospective, multicenter, randomized, controlled pivotal trial is to evaluate safety and effectiveness in patients with degenerative mitral regurgitation (DMR) who have been determined to be at prohibitive risk for mitral valve surgery by the Heart Team.
of patients sustained MR ≤ 1+ at 2 years with the PASCAL system1

freedom from cardiovascular mortality at 2 years with the PASCAL system

freedom from HFH at 2 years with the PASCAL system

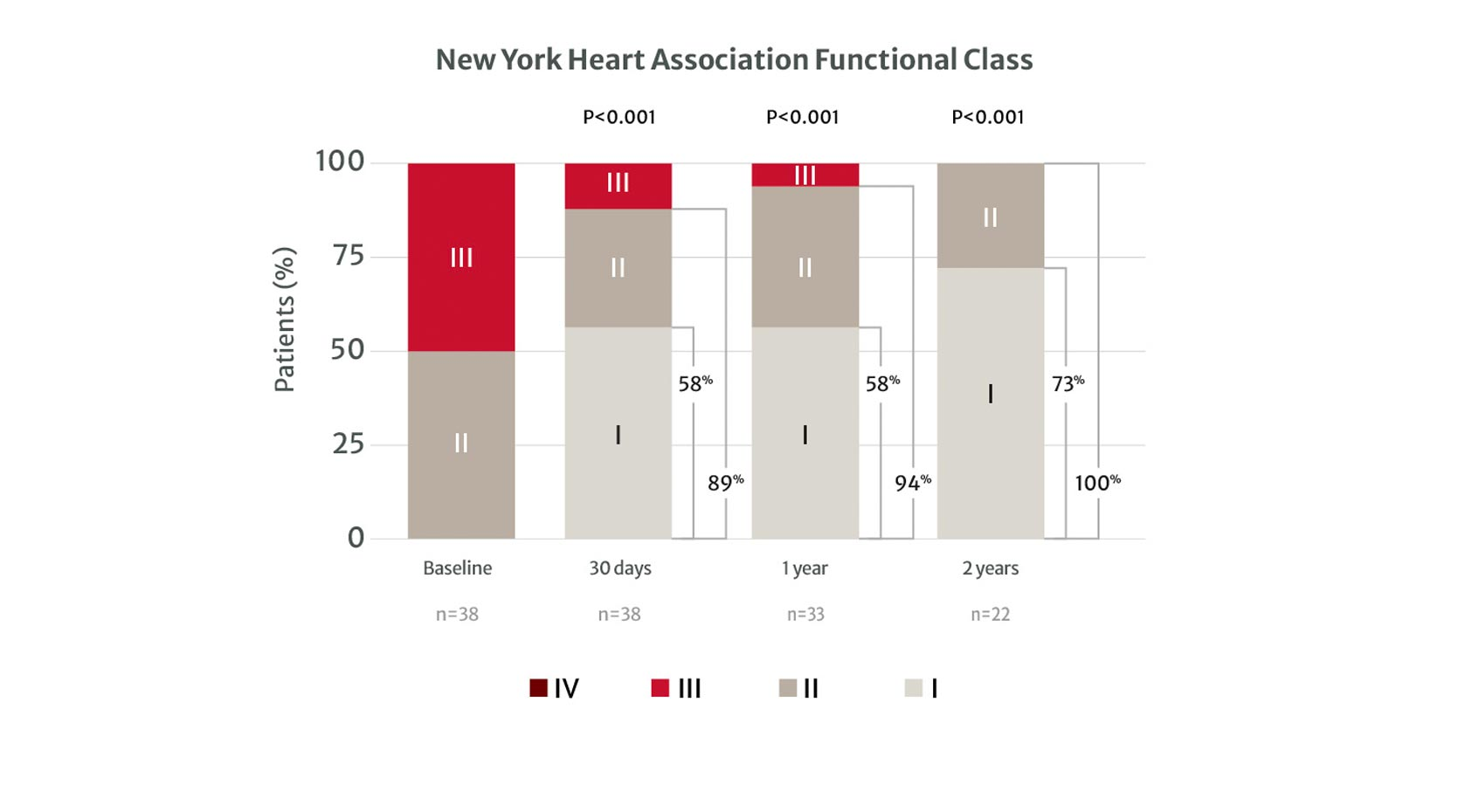
of patients achieved NYHA Class I/II at 2 years with the PASCAL system
Graph shows unpaired analysis for NYHA functional class (mean ±SD). P values for intragroup comparison for NYHA were calculated from paired analysis using the Wilcoxon signed rank test. P values for intergroup comparison of NYHA functional class I/II were calculated using Fisher exact test.

point improvement from baseline to 2 years with the PASCAL system
Graph shows unpaired analysis for KCCQ (mean ±SD). P value for intergroup comparison of KCCQ score was calculated using the analysis of covariance (ANCOVA) model adjusted for baseline values and planned treatment as covariates.


MR Reduction

Freedom from cardiovascular mortality

Freedom from heart failure hospitalization

New York Heart Association Functional Class

KCCQ Score
of patients sustained MR ≤ 1+ at 2 years with the PASCAL system1
freedom from cardiovascular mortality at 2 years with the PASCAL system
freedom from HFH at 2 years with the PASCAL system
of patients achieved NYHA Class I/II at 2 years with the PASCAL system
Graph shows unpaired analysis for NYHA functional class (mean ±SD). P values for intragroup comparison for NYHA were calculated from paired analysis using the Wilcoxon signed rank test. P values for intergroup comparison of NYHA functional class I/II were calculated using Fisher exact test.
point improvement from baseline to 2 years with the PASCAL system
Graph shows unpaired analysis for KCCQ (mean ±SD). P value for intergroup comparison of KCCQ score was calculated using the analysis of covariance (ANCOVA) model adjusted for baseline values and planned treatment as covariates.
1. Zahr F, et al. CLASP IID Randomized Trial and Registry: Two-Year Outcomes of Transcatheter Edge-to-Edge Repair for Degenerative Mitral Regurgitation. Presented at: TCT Annual Congress; 2024 Oct 30; Washington, DC.

CLASP IID is a clinical trial from Edwards Lifesciences, reinforcing TEER as safe and effective for the treatment of patients with degenerative mitral regurgitation.
The objective of this prospective, multinational registry within the construct of the CLASP IID trial is to study significant symptomatic DMR patients with complex mitral valve anatomy deemed suitable for treatment with the PASCAL system.
of patients sustained MR ≤2 + at 2 years with the PASCAL system

freedom from cardiovascular mortality at 2 years with the PASCAL system

KCCQ improvement from baseline to 2 years with the PASCAL system

of patients achieved NYHA Class I/II at 2 years with the PASCAL system

MR Reduction

Freedom from cardiovascular mortality

KCCQ Score

of patients sustained MR ≤2 + at 2 years with the PASCAL system
freedom from cardiovascular mortality at 2 years with the PASCAL system
KCCQ improvement from baseline to 2 years with the PASCAL system
of patients achieved NYHA Class I/II at 2 years with the PASCAL system
1. Zahr F, et al. CLASP IID Randomized Trial and Registry: Two-Year Outcomes of Transcatheter Edge-to-Edge Repair for Degenerative Mitral Regurgitation. Presented at: TCT Annual Congress; 2024 Oct 30; Washington, DC.
The first, international multicenter study to evaluate the PASCAL system provides 3-year outcomes in patients with clinically significant MR. The results in this section are for the patients in this study with degenerative mitral regurgitation.
of DMR patients sustained MR ≤ 2+ at 3 years with the PASCAL system2

freedom from all-cause mortality at 3 years with the PASCAL system

freedom from HFH at 3 years with the PASCAL system

of DMR patients achieved NYHA Class I/II at 3 years with the PASCAL system


DMR

Survival

Freedom from HF rehospitalization

New York Heart Association Functional Class
of DMR patients sustained MR ≤ 2+ at 3 years with the PASCAL system2
freedom from all-cause mortality at 3 years with the PASCAL system
freedom from HFH at 3 years with the PASCAL system
of DMR patients achieved NYHA Class I/II at 3 years with the PASCAL system
2. Spargias K et al. Three-year outcomes for the transcatheter repair in patients with mitral regurgitation from the CLASP study. CCI. 2023.
