Objective
The objective of this study was to validate the results of the STS Database study and evaluate costs associated with external aortic clamping (EAC) versus endo-aortic balloon occlusion (EABO) using the Premier Healthcare Database.

Real-world evidence comparing hospitalization cost and clinical outcomes evaluation between the endo-aortic balloon and external aortic clamp in cardiac surgery1
The objective of this study was to validate the results of the STS Database study and evaluate costs associated with external aortic clamping (EAC) versus endo-aortic balloon occlusion (EABO) using the Premier Healthcare Database.

A 3:1 propensity score- and exact-matched cohort of 1,663 EABO-eligible cardiac surgery cases (10/2015 to 03/2020) was extracted from the Premier database. The researchers examined cost outcomes and clinical outcomes using multivariable generalized linear models to detect differences between groups.

There was no statistically significant difference in total hospitalization costs between EABO versus EAC patient stays. Length of stay was significantly shorter for the EABO group (median (IQR): 6 (5-8) days vs. 7 (5-10) days, P < 0.0001). Rates of myocardial infarction and postcardiotomy syndrome were significantly lower in patients with EABO versus EAC, with no significant differences in any other clinical outcomes.
This real-world evidence suggests that EABO has similar costs and clinical outcomes as the EAC.
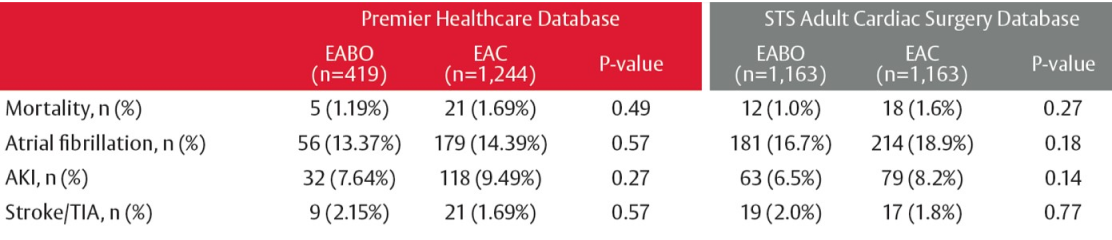
Table 1: Comparison of the Premier database to the STS database analysis

The authors compare outcomes and hospital costs of endo-aortic balloon occlusion (EABO) versus external aortic clamping (EAC) in patients undergoing minimally invasive cardiac surgery in a large administrative database.
Endo-aortic balloon occlusion is associated with shorter bypass and length-of-stay times in MIS mitral surgery with similar safety and effectiveness.
In an analysis of the STS Adult Cardiac Surgery Database, endo-aortic balloon occlusion was associated with:
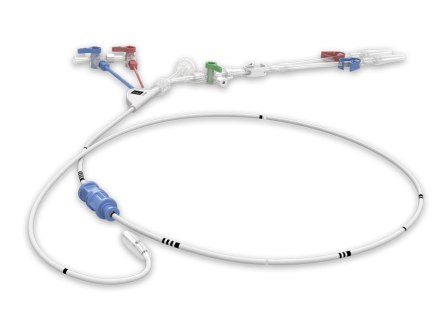
The IntraClude device is an integrated system to occlude, arrest, and vent the heart in addition to monitoring aortic root pressure.

CAUTION: Federal (United States) law restricts this device to sale by or on the order of a physician. See instructions for use for full prescribing information, including indications, contraindications, warnings, precautions, and adverse events.
We are committed to providing your institution, clinicians and staff with the highest levels of customer service and support to ensure seamless product implementation and ongoing use, including:
24/7 Technical support
For product information and orders