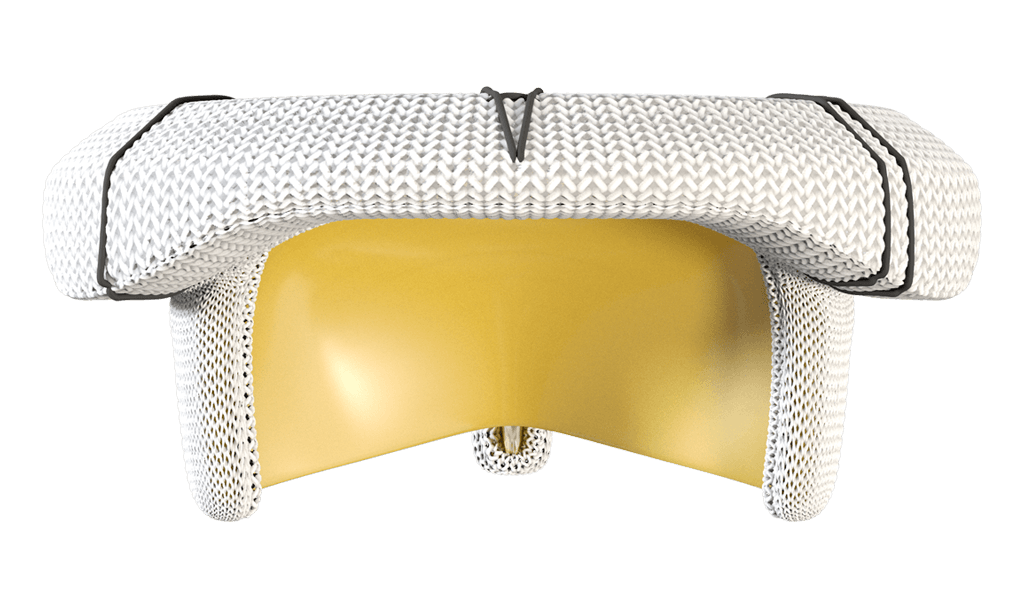
MITRIS RESILIA mitral valve

MITRIS RESILIA:* designed to handle the pressure of the mitral position
Mitral regurgitation is one of the most common valvular heart diseases, predominantly affecting patients ≥65 years of age.1-3 If left untreated, moderate-severe mitral regurgitation is associated with excess morbidity and mortality.4
In addition, the median age for mitral valve replacement patients is relatively young, at 66 years.5 Combined with an increasing life expectancy,6 it is more important than ever that there’s a tissue valve built to last for patients in the long-term.
Edwards Lifesciences brings you the MITRIS RESILIA valve, designed to handle the pressure of the mitral position.
Key features of the MITRIS valve

Cobalt chromium bands provide good visibility under fluoroscopy
For easy identification of the landing zone for potential future transcatheter interventions.

Dry storage
To eliminate need for rinsing.
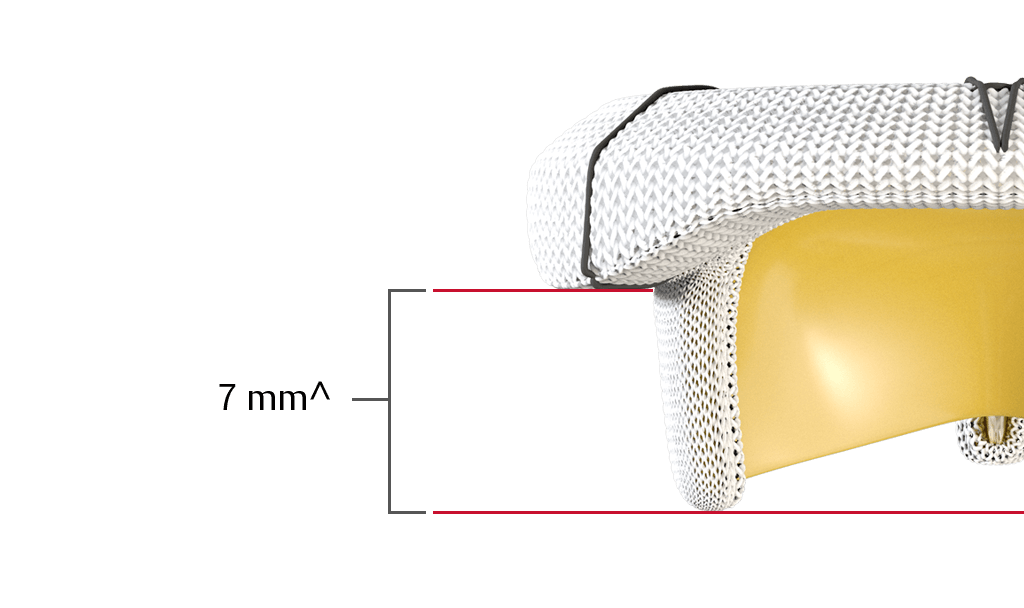
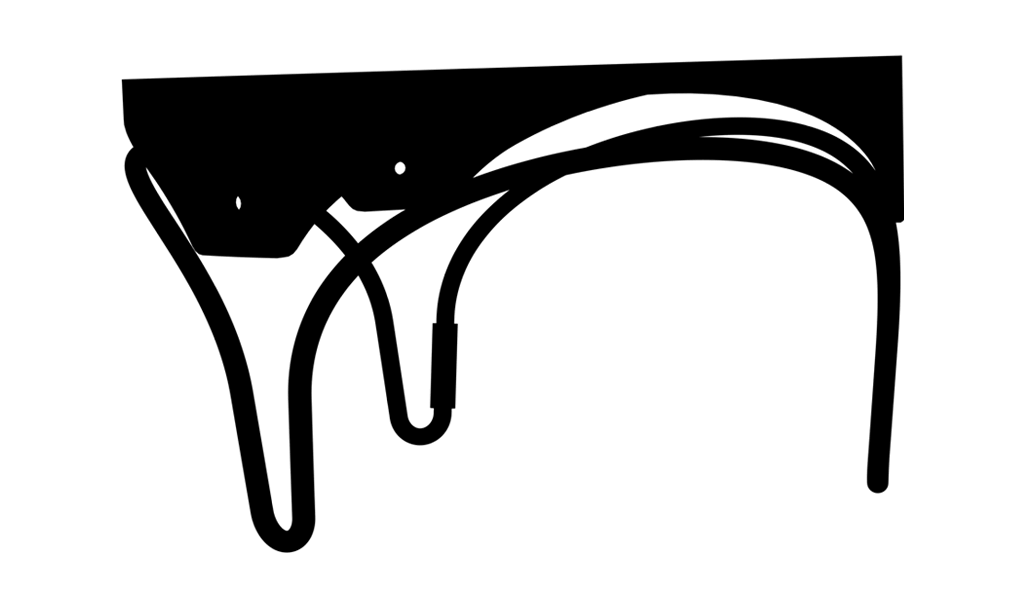
Lowest anterior profile stents
Do not obstruct blood flow through the left ventricular outflow tract (LVOT).
^On a label size 25 mm. Anterior Profile Height.
Device preparation animation
Incorporating RESILIA tissue technology
RESILIA tissue is bovine pericardial tissue treated with a special integrity preservation technology that mitigates free aldehydes, a key factor in tissue calcification.7 The tissue is also stored dry to prevent further glutaraldehyde exposure.
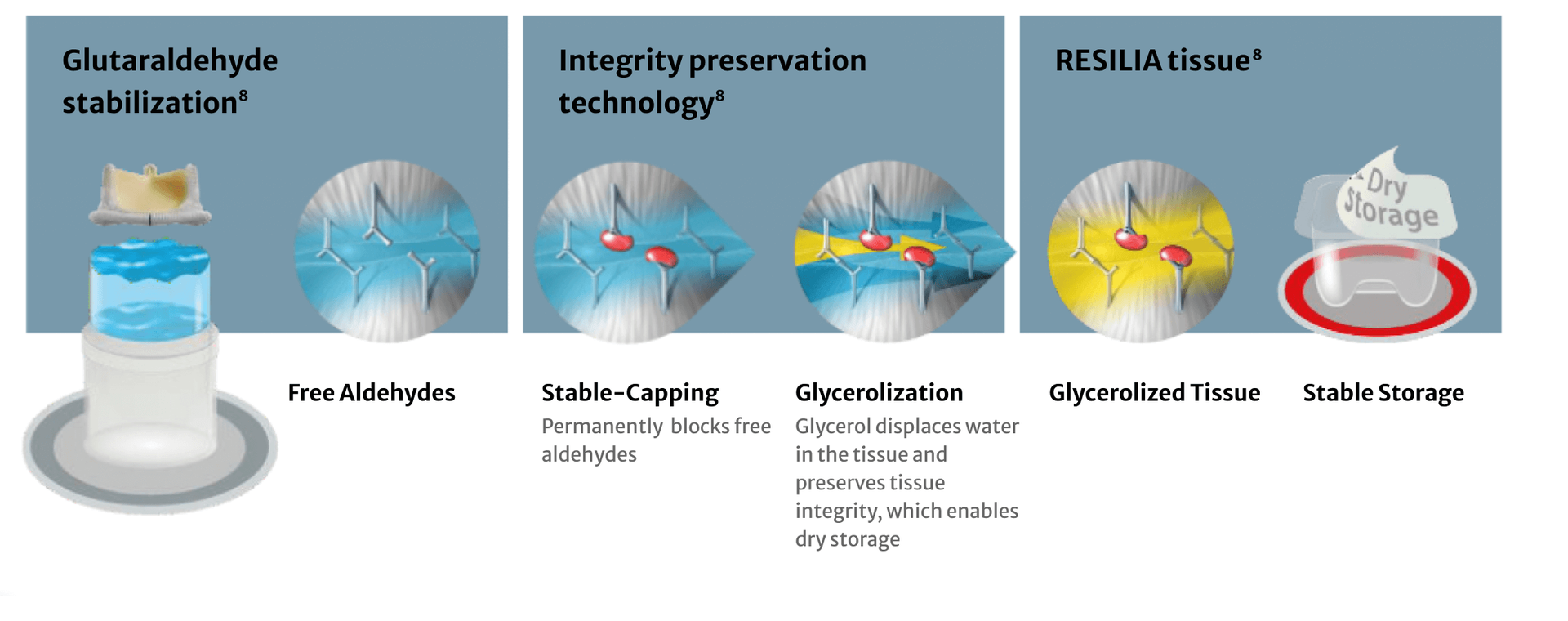
The MITRIS RESILIA valve is designed to offer enhanced tissue anticalcification technology that will potentially allow the valve to last longer
RESILIA tissue demonstrates significantly less calcium compared to other bovine tissues, offering the possibility of delaying potential surgical re-interventions later in life.9
An enhanced delivery experience
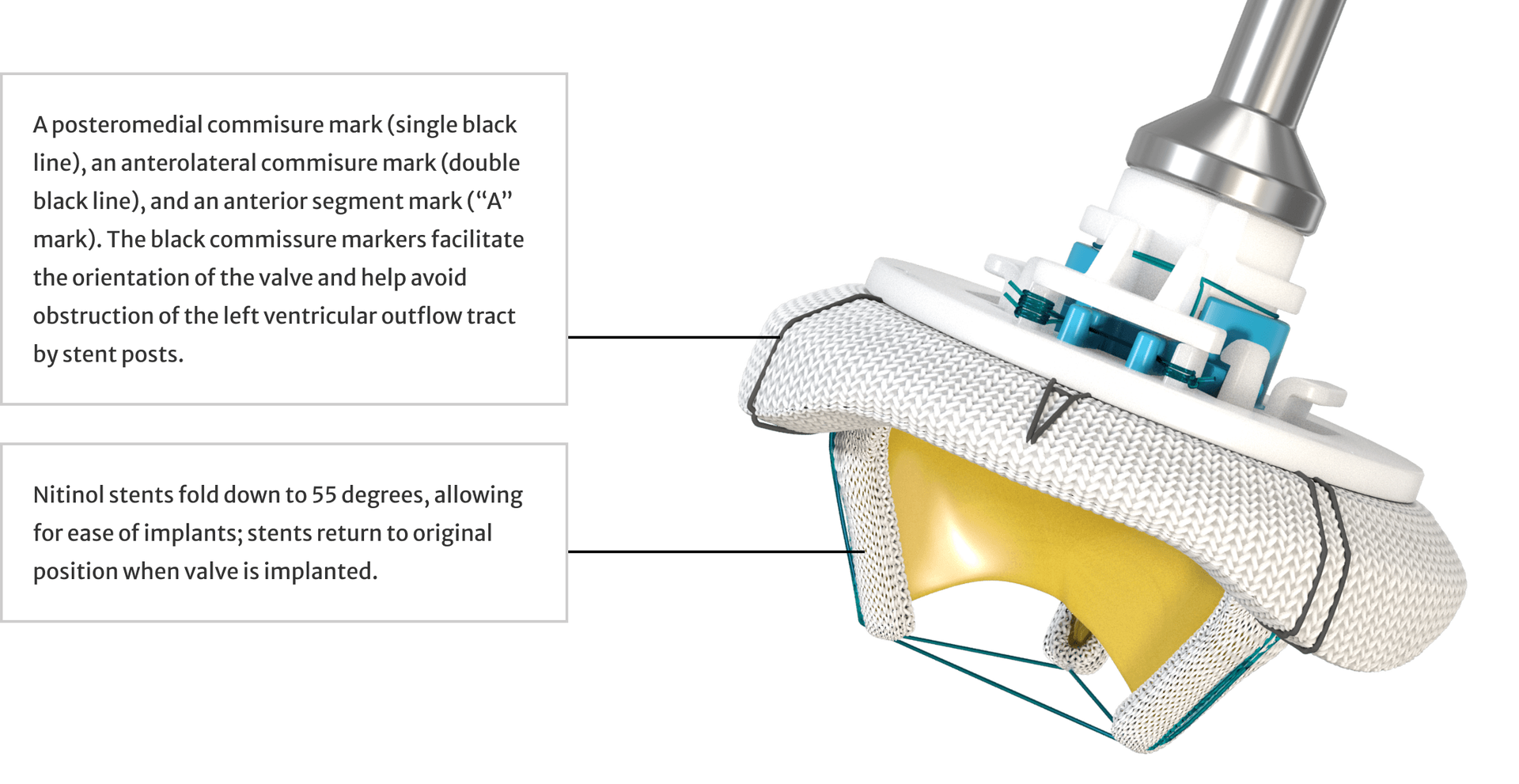
Indications for use8
The MITRIS RESILIA mitral valve, Model 11400M is indicated for the replacement of native or prosthetic mitral heart valves.
Key materials list
- Valve leaflets: Bovine pericardium
- Stent: Cobalt-chromium alloy and polyester band with a nitinol wireform
- Fabric stent covering: Polyester and Polytetrafluoroethylene (PTFE) cloth
- Valve sewing ring: Silicone rubber
Tissue platform
The MITRIS valve features RESILIA tissue and is treated with a special integrity preservation technology that effectively eliminates free aldehydes while protecting and preserving tissue.
No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients. Additional clinical data for up to 10 years of follow-up are being collected to monitor the long-term safety and performance of RESILIA tissue.
General product information
- Storage temperature: 10°C to 25°C
- Storage: Dry storage
- Rinse procedure: None required
References
- Singh JP, et al. Am J Cardiol. 1999;83(6):897–902.
- Nkomo VT, et al. Lancet. 2006;368(9540):1005–11.
- De Backer OD, et al. Circ Cardiovasc Interv. 2014;7(3):400–9.
- Dziadzko V, et al. Lancet. 2018;391(10124):960–9.
- Romano M, et al. J Thorac Cardiovasc Surg. 2023:S0022–5223(23)00968-6.
- World Health Organisation. GHE: Life expectancy and healthy life expectancy. Available at: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-life-expectancy-and-healthy-life-expectancy. Accessed June 2024.
- Flameng W, et al. J Thorac Cardiovasc Surg. 2015;149:340–45.
- Edwards Lifesciences. MITRIS RESILIA Mitral Valve, Model 11400M, Instructions for use.
- Heimansohn DA, et al. JTCVS Open. 2023;15:151–163.
*No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients. Additional clinical data for up to 10 years of follow-up are being collected to monitor the long-term safety and performance of RESILIA tissue.
Medical device for professional use
For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).


