
An alliance to advance patient care

Minimally-invasive, advanced haemodynamic insights: Available for your Philips IntelliVue monitors*
As part of an innovation-driven partnership between Edwards Lifesciences and Philips, FloTrac sensor is available on compatible Philips IntelliVue monitors.* Together, Edwards’ trusted solution for advanced haemodynamic monitoring and Philips’ market-leading monitors deliver advanced insights into patient status and support clinical decision-making.
*FloTrac sensor is compatible with Philips IntelliVue MX500, MX550, MX750 and MX850 monitors.
Unlock proactive decision support with continuous haemodynamic insights
Continuous clarity provided by the minimally-invasive FloTrac sensor offers proactive decision support to manage haemodynamic instability and ensure adequate patient perfusion.


FloTrac sensor delivers:
- Stroke volume (SV)
- Stroke volume variation (SVV)
- Mean arterial pressure (MAP)
- Cardiac output (CO)
- Systemic vascular resistance (SVR)

Guide individualised treatment with the trusted solution for minimally-invasive haemodynamic monitoring
Proactive decision support offered by FloTrac sensor helps guide individualised treatment decisions for your moderate- to high-risk surgery patients.
FloTrac sensor can also be utilised perioperatively to proactively manage your patient’s physiological status in rapidly changing clinical situations.
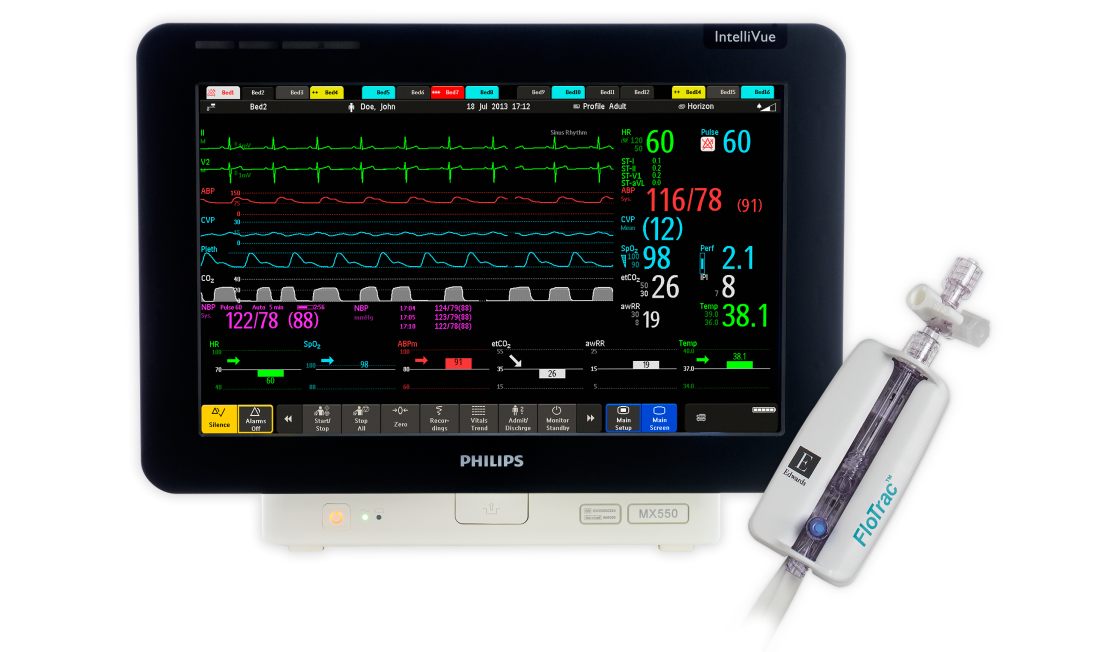
Quick access to advanced haemodynamic insights with plug-and-play integration
Plug into a legacy of haemodynamic innovation and expand your monitoring capabilities.
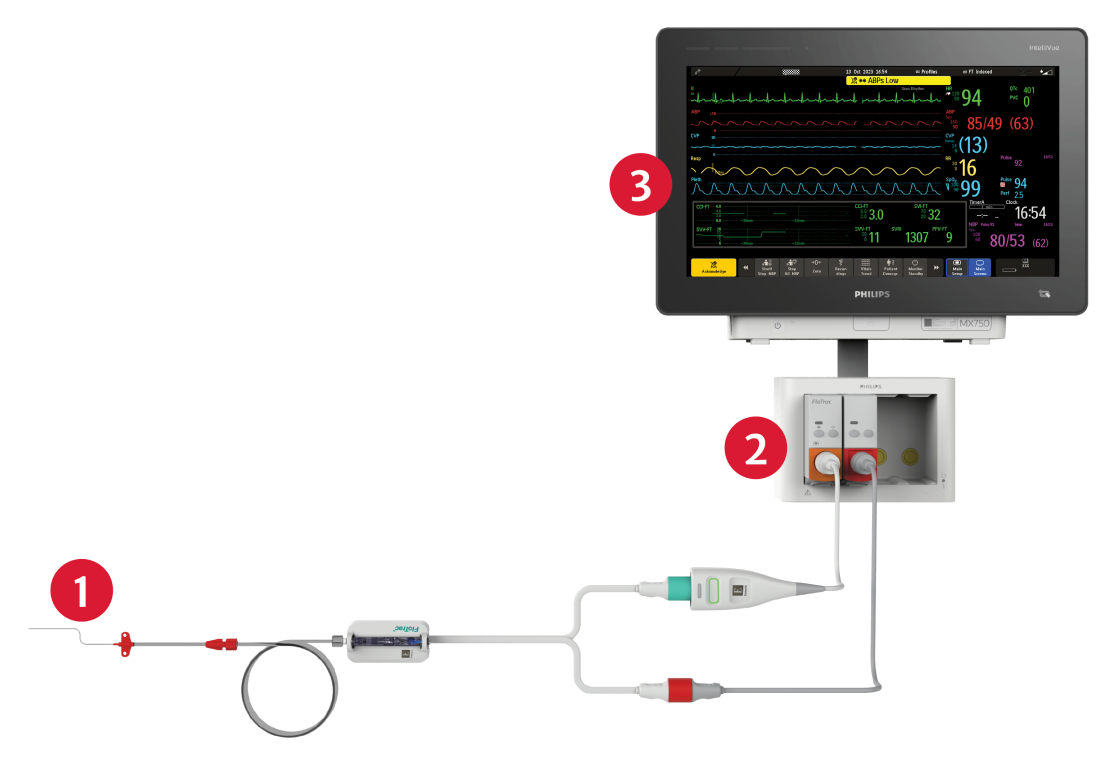
- FloTrac sensor seamlessly connects to an existing arterial catheter.
- Plug-and-play integration allows for quick access to the insights you need.
- FloTrac sensor trends and parameters are displayed on your Philips IntelliVue monitor for advanced insights into patient status.
Access trusted haemodynamic insights through a partnership empowering individualised care.


Medical device for professional use
Medical device for professional use
For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).

