SAPIEN 3 Ultra transcatheter heart valve


Built upon the proven SAPIEN platform, the SAPIEN 3 Ultra transcatheter heart valve is designed with your patient's future needs in mind.
Taller, textured outer skirt1
- Approximately 40% increased outer skirt*
- Textured PET material
- 14F sheath compatibility
*Compared to the Edwards SAPIEN 3 valve
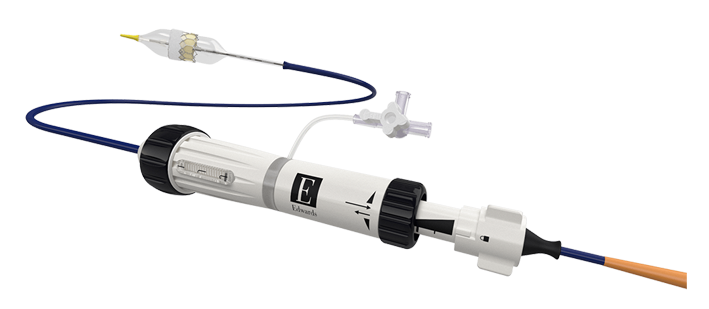
Edwards Commander Delivery System: For predictable, proven results2
Delivering your strategy for lifetime management
through the eSheath+ Introducer Set
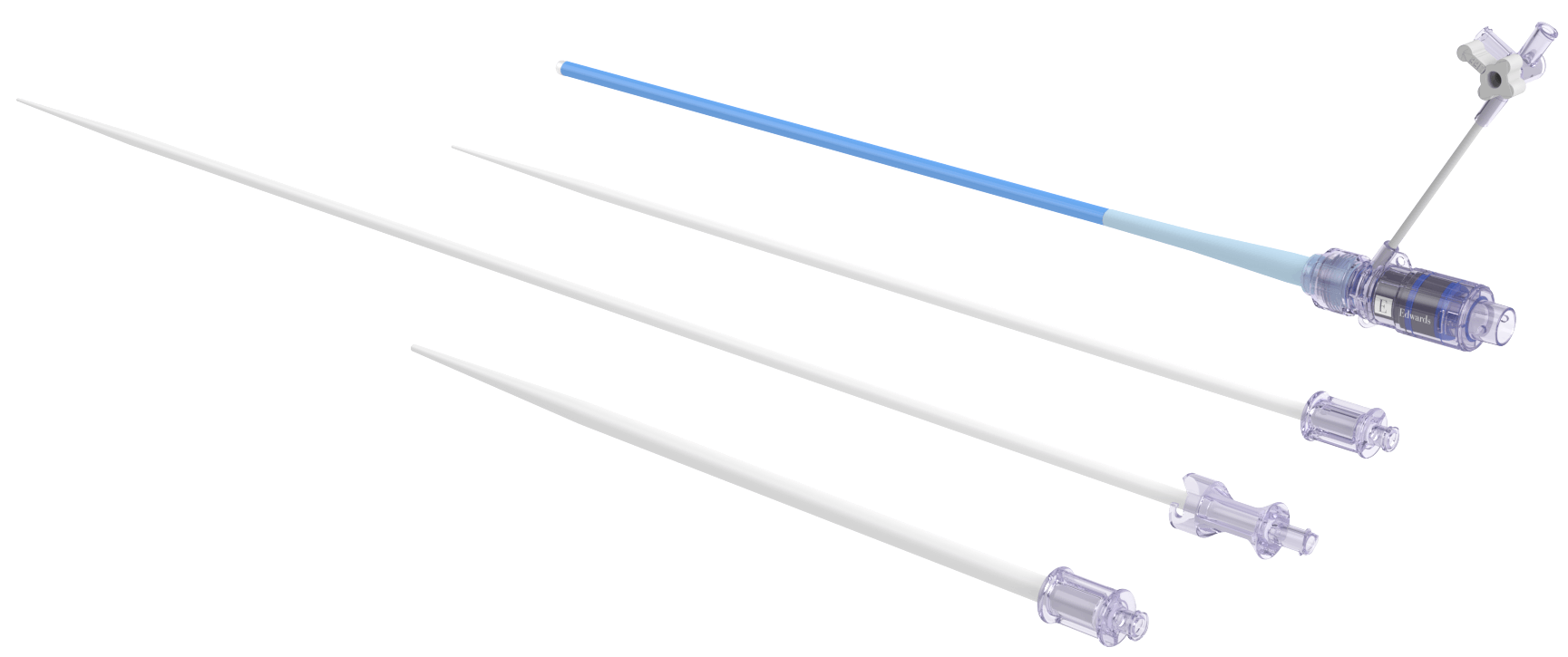
- Consistently reduce current and future patient complications
(i.e vascular bleeding)3,4 - Improve and simplify the implanter's experience†
- Accelerate the preparation phase for the Heart Team†
TAVI procedural animation using the SAPIEN 3 Ultra system
Learn more about SAPIEN 3 Ultra TAVI* clinical evidence
*TAVI = Transcatheter Aortic Valve Implantation
References
1. Nazif,et al. Real-World Experience With the SAPIEN 3 Ultra Transcatheter Heart Valve. 2021
2. Webb J, Gerosa G, Lefèvre T, et al. Multicenter evaluation of a next-generation balloon-expandable transcatheter aortic valve. J Am Coll Cardiol. 2014;64(21):2235-2243.
3. Thourani VH, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet. 2016 May 28;387(10034):2218-25)
4. Mack MJ, et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med. 2019 May 2;380(18):1695-1705)
5. Mack MJ, et al. Transcatheter Aortic-Valve Replacement in Low-Risk Patients at Five Years. N Engl J Med. 2023 Nov 23;389(21):1949-1960.
6. Madhavan MV, et al. Outcomes of SAPIEN 3 Transcatheter Aortic Valve Replacement Compared With Surgical Valve Replacement in Intermediate-Risk Patients. J Am Coll Cardiol.
2023 Jul 11;82(2):109-123.
†Data on file at Edwards Lifesciences
Medical device for professional use.
For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
Edwards, Edwards Lifesciences, the stylized E logo, Commander, Edwards Commander, eSheath, eSheath+, SAPIEN, SAPIEN 3, SAPIEN 3 Ultra, and ThermaFix are trademarks or service marks of Edwards Lifesciences Corporation or its affiliates. All other trademarks are the property of their respective owners.
PP--EU-8241 v1.0
Edwards Lifesciences Sàrl • Route de l’Etraz 70, 1260 Nyon, Switzerland • edwards.com
