Communiqués de presse

Edwards SAPIEN 3 Valve Proves Superior To Surgery In Partner 3 Trial
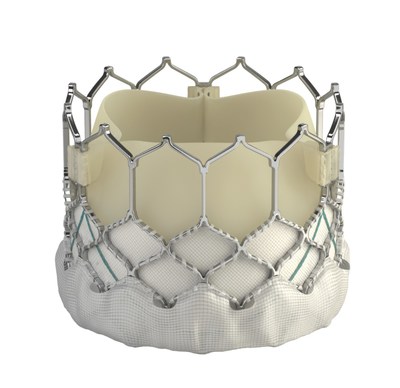
NEW ORLEANS, March 16, 2019 -- Edwards Lifesciences Corporation (NYSE: EW), the global leader in patient-focused innovations for structural heart disease and critical care monitoring, today announced that the results of the randomized PARTNER 3 Trial demonstrated superiority for the SAPIEN 3 transcatheter aortic valve over outcomes with surgery.
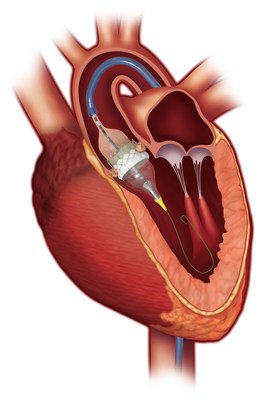
The trial, which compared treatment with the SAPIEN 3 valve to surgery in patients with severe symptomatic aortic stenosis (AS) at low risk of death from surgery, achieved superiority of its primary endpoint at one year. The results of the trial will be presented on Sunday as part of the late-breaking clinical trials at the American College of Cardiology's 68th Annual Scientific Session (ACC.19) in New Orleans, and have been published online in the New England Journal of Medicine.
The PARTNER 3 Trial was an independently evaluated, randomized clinical trial comparing outcomes between transcatheter aortic valve replacement (TAVR) and open-heart surgery in this patient group. TAVR with the SAPIEN 3 valve achieved superiority, with a 46 percent reduction in the event rate for the primary endpoint of the trial, which was a composite of all-cause mortality, all stroke and rehospitalization at one year.
"From the first TAVR case more than 17 years ago, to the extensive series of rigorous clinical trials to date, the PARTNER studies have shown increasingly robust outcomes with TAVR and have supported the treatment of hundreds of thousands of patients globally. This demonstrates that TAVR is a proven therapy for severe aortic stenosis, a deadly and debilitating disease that has a worse outlook than many cancers," said Martin B. Leon, M.D., director of the Center for Interventional Vascular Therapy at NewYork-Presbyterian/Columbia University Medical Center and professor of medicine at the Columbia University College of Physicians and Surgeons. Leon is the national co-principal investigator of the PARTNER 3 Trial. "In the PARTNER 3 Trial, TAVR with the SAPIEN 3 valve showed a remarkably low death and disabling stroke rate of 1.0 percent at one year versus 2.9 percent for surgery. Based upon these clinical trial findings at one year, TAVR should be considered the preferred therapy in low surgical risk aortic stenosis patients."
The PARTNER 3 Trial randomized 1,000 patients at 71 centers between March 2016 and October 2017. Patients were assigned to undergo either TAVR with the SAPIEN 3 valve or surgery with any commercially available surgical valve. All patients were followed for at least one year, and 10-year clinical and echocardiographic follow-up is planned for all patients.
"These data demonstrate that TAVR with the SAPIEN 3 valve gives low surgical risk patients with severe AS a treatment with better outcomes, less time in the hospital and the ability to resume their everyday lives more quickly," said Larry Wood, corporate vice president, transcatheter aortic valve replacement. "It is also highly encouraging to see that 96 percent of TAVR patients were discharged to home as opposed to remaining in a hospital or specialized care setting."
More than 600,000 patients around the world have benefitted from TAVR. The SAPIEN 3 valve is approved in the United States for the treatment of intermediate and higher risk patients with severe, symptomatic AS; it is not yet approved for the treatment of low-risk patients. Edwards continues to anticipate U.S. Food and Drug Administration approval of a low-risk indication for the SAPIEN 3 valve late this year.
Endpoint | 30 Days | p-value‡ | 1 Year | p-value‡ | ||
TAVR (N=496) SAVR(N=454) | TAVR (N=496) SAVR(N=454) | |||||
All-cause death, | 4.2% | 9.3% | 0.002 | 8.5% | 15.1% | 0.001 |
All-cause death | 0.4% | 1.1% | 0.21 | 1.0% | 2.5% | 0.09 |
All stroke | 0.6% | 2.4% | 0.02 | 1.2% | 3.1% | 0.04 |
Death or disabling | 0.4% | 1.3% | 0.12 | 1.0% | 2.9% | 0.03 |
Rehospitalization† | 3.4% | 6.5% | 0.04 | 7.3% | 11.0% | <0.05 |
† Rehospitalization (valve-related or procedure-related and including heart failure) ‡ p-values is based on a log-rank test All event rates are Kaplan-Meier estimates | ||||||
About Edwards Lifesciences
Edwards Lifesciences, based in Irvine, Calif., is the global leader in patient-focused medical innovations for structural heart disease, as well as critical care and surgical monitoring. Driven by a passion to help patients, the company collaborates with the world's leading clinicians and researchers to address unmet healthcare needs, working to improve patient outcomes and enhance lives. For more information, visit www.Edwards.com and follow us on Twitter @EdwardsLifesci.
This news release includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. These forward-looking statements include, but are not limited to, Mr. Wood's and Dr. Leon's statements and statements regarding the expected FDA approval, overall therapy benefits and expected patient outcomes. Forward-looking statements are based on estimates and assumptions made by management of the company and are believed to be reasonable, though they are inherently uncertain and difficult to predict. Our forward-looking statements speak only as of the date on which they are made and we do not undertake any obligation to update any forward-looking statement to reflect events or circumstances after the date of the statement.
Forward-looking statements involve risks and uncertainties that could cause results to differ materially from those expressed or implied by the forward-looking statements based on a number of factors, including but not limited to, unexpected delays or changes in the regulatory approval, unanticipated outcomes of clinical experience with the product following longer term clinical experience, or unanticipated manufacturing, legal, quality or regulatory delays or issues. These factors are detailed in the company's filings with the Securities and Exchange Commission including its Annual Report on Form 10-K for the year ended December 31, 2018. These filings, along with important safety information about our products, may be found at edwards.com.
Edwards, Edwards Lifesciences, the stylized E logo, Edwards SAPIEN, SAPIEN, and SAPIEN 3 are trademarks of Edwards Lifesciences Corporation and its affiliates. All other trademarks are the property of their respective owners. This statement is made on behalf of Edwards Lifesciences Corporation and its subsidiaries.

Personnes-ressources
Investisseurs
Médias
Amy Meshulam
(Vice-président senior, Communications mondiales)
