Promising data. Inspiring results.

Edwards Lifesciences is committed to continued scientific advancement, and studies that investigate the safety and performance of new technologies.
Our trials aim to build upon one another by adding new variables which further challenge valve safety and efficacy. Our COMMENCE trial increased the patient population from the EU Feasibility trial, and the RESILIENCE trial is designed to look at different outcome measures to help further establish long-term valve durability.
Learn more about the current methodologies, promising results and conclusions of the various RESILIA tissue studies below.
RESILIA tissue is building a track record of study data.
| Start Year | Description |
| 2010 | Juvenile sheep study |
| 2011 | EU human feasibility study in Poland |
| 2012 | Aortic/mitral COMMENCE IDE study |
| 2017 | INSPIRIS RESILIA valve approved by FDA |
| 2018 | RESILIENCE study begins |
Pannus Formation Study
Pannus Formation Study Clinical Summary

Methods
- This publication reports the outcomes of two independent studies using a juvenile sheep model of mitral valve replacement with bovine pericardial tissue
- In an 8-month study, valves with RESILIA tissue were compared to control valves with XenoLogiX treatment (XLX)
- In a 5-month study, valves with RESILIA tissue were compared to control valves treated with the ThermaFix process (TFX)
- Control valves were commercially available Carpentier-Edwards PERIMOUNT mitral valves, models 6900P, and 7000TFX. Test articles were the same models configured with RESILIA tissue
- Explanted valves were examined macroscopically and histologically. Histological observations were made by an independent pathologist, blinded to group identity
- Independent means of pannus quantification were employed in the two studies
For a more detailed overview of the Pannus Formation Study methodology and results please download the clinical summary.
Pannus Formation Study Clinical Summary

Juvenile Sheep Study
Juvenile Sheep Study Clinical Paper Details

Methods
- 45 juvenile sheep were randomized and either a PERIMOUNT mitral valve (6900P, control group) or the same valve design incorporating the RESILIA tissue preservation technology (test group) was implanted in the mitral position
- All valves were 25 mm
- A transthoracic echocardiography was performed at 1 week and at 8 months postoperatively
- Necropsy was performed at 8 months, and the valves were examined radiographically (soft tissue radiograph), histologically (hematoxylin and eosin and Von Kossa staining), and chemically (calcium content)
For a more detailed overview of the Juvenile Sheep Study methodology and results, please see link to clinical paper details.
Juvenile Sheep Study Clinical Paper Details

EU Feasibility Study
EU Feasibility Study Clinical Summary
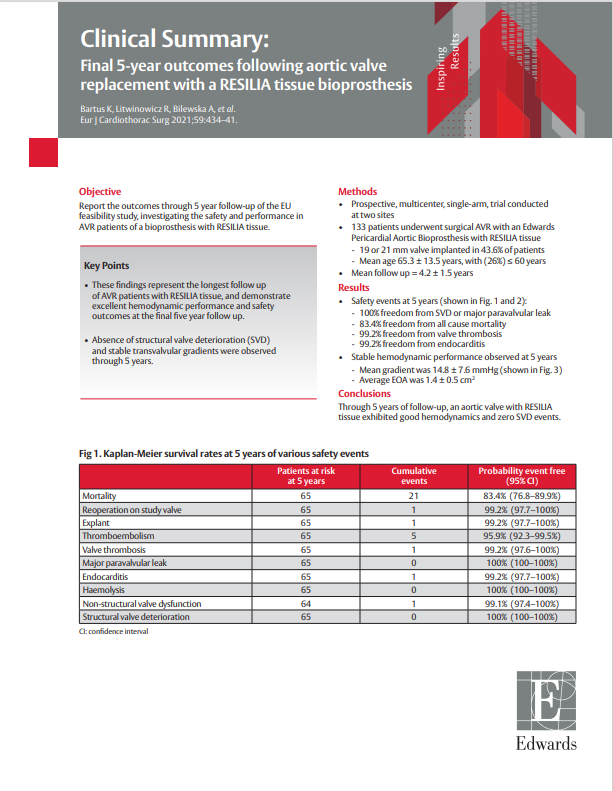
Methods
- Prospective, multicenter, single-arm, trial conducted at two sites
- 133 patients underwent surgical AVR with an Edwards Pericardial Aortic Bioprosthesis with RESILIA tissue
- Mean age 65.3 ± 13.5 years, with (26%) ≤ 60 years
- 19 or 21 mm valve implanted in 43.6% of patients
- Median follow up = 5 years; Total late follow-up time of 565.2 patient years (LPY)
For a more detailed overview of the EU Feasibility Study methodology and results please download the clinical summary.
EU Feasibility Study Clinical Summary

COMMENCE Trial
Methods
COMMENCE Trial Clinical Summary

- Prospective, multinational, multicenter (n = 27), single-arm, FDA Investigational Device Exemption trial
- 689 patients underwent surgical AVR with the Edwards Pericardial Aortic Bioprosthesis with RESILIA tissue (model 11000A)
- Mean age 66.9 ± 11.6 years, with 140 patients (21%) under 60 years
- 71.8% male
- 26% NYHA Class III/IV
- Mean STS PROM 2.0 ± 1.8%
- 59% isolated AVR
- 2989 aggregate patient-years of follow up
- Follow up: 4.3 ± 1.4 years (n=471)
For a more detailed overview of the COMMENCE trial methodology and results please download the clinical summary.
COMMENCE Trial Clinical Summary

Five-year outcomes of the COMMENCE Trial investigating Aortic Valve Replacement with a Novel Tissue Bioprosthesis
Hear the results of the COMMENCE trial conducted to evaluate the safety and effectiveness of aortic valve replacement (AVR) using a bioprosthesis with novel RESILIA tissue. Presenting Author: Joseph Bavaria, MD
COMMENCE Trial Hemodynamics Sub-Analysis
Sub-analysis of the five-year outcomes of the COMMENCE aortic trial investigating patient and valve factors associated with longitudinal hemodynamic change and the study valve’s hemodynamic performance.5
COMMENCE Trial Hemodynamics Sub-Analysis Clinical Summary

Methods
- Patients underwent echocardiograms annually through 5 years of follow up; these were evaluated by a core laboratory.
- Only patients with evaluable echo data (N=663) at any of the post-op visits were eligible for inclusion in this sub-analysis.
- Longitudinal models were constructed to estimate the change in mean gradient and change in effective orifice area (EOA) separately over 5 years post-operatively.
For a more detailed overview of the COMMENCE trial hemodynamics sub-analysis methodology and results, download the clinical summary.
COMMENCE Trial Hemodynamics Sub-Analysis Clinical Summary

COMMENCE Trial Bicuspid Aortic Valve Sub-Analysis
Sub-analysis of the five-year outcomes of the COMMENCE aortic trial investigating safety outcomes and mean gradients in patients with bicuspid aortic valves.6
COMMENCE Trial Bicuspid Aortic Valve Sub-Analysis Clinical Summary

Methods
- Patients underwent echocardiograms annually through 5 years of follow up; these were evaluated by a core laboratory.
- Evaluations included safety outcomes, paravalvular and transvalvular regurgitation, and summary hemodynamics.
- Longitudinal models were constructed to estimate the change in mean gradient and change in effective orifice area (EOA) separately over 5 years post-operatively.
For a more detailed overview of the COMMENCE trial bicuspid aortic valve sub-analysis methodology and results, download the clinical summary.
COMMENCE Trial Bicuspid Aortic Valve Sub-Analysis Clinical Summary

COMMENCE Mitral Trial
Four-year outcomes of the COMMENCE trial investigating mitral valve replacement (MVR) with a novel tissue bioprosthesis showed clinically stable hemodynamics and one incident of SVD in 82 patients. In the one instance of SVD reported, the patient, a 77-year-old female, had multiple comorbidities including: renal cell carcinoma, coronary artery disease, hypertension, atrial fibrillation, non-Hodgkin’s lymphoma after bone marrow transplant and ongoing tobacco use.
RESILIENCE Study Design
Study Design of the Prospective Non-Randomized Single Arm Multicenter Evaluation of the Durability of Aortic Bioprosthetic Valves with RESILIA Tissue in Subjects under 65 Years Old (RESILIENCE Trial)7
RESILIENCE Study Design Clinical Summary

The objective of the RESILIENCE trial is to determine the time to valve failure due to valve deterioration requiring re-intervention, as well as to collect/investigate early potential predictors of valve durability (e.g. calcification and hemodynamic deterioration) in RESILIA tissue. The RESILIENCE trial is the first prospective study to associate both clinical and imaging definitions of SVD with long-term (11 years) bioprosthetic valve durability.
RESILIENCE Study Design Clinical Summary

Methods
- Multicenter, prospective, non-randomized, single-arm, observational trial
- Up to 250 patients who previously underwent SAVR with a RESILIA tissue valve will be enrolled at up to 15 investigational centers across the United States and Europe
- Includes patients <65 years old; at time of implant this is a population that is at high risk to develop SVD
- First patient enrolled on November 21, 2018
- Anticipated 3 year enrollment period
- Patients undergo a TTE and non-contrast CT at 5, 7, 9, and 11 years post-valve implant (Figure 1).
- The definition proposed by Dvir et al. includes four stages: Stage 0: no SVD; Stage 1: Morphological SVD; Stage 2: Moderate hemodynamic SVD; and Stage 3: Severe hemodynamic SVD


Echocardiography and CT Core Laboratories will be responsible for independently evaluating TTEs and CTs submitted by trial sites and will report upon the primary and secondary outcomes of the trial.
- The morphology and mobility of the bioprosthetic valve leaflets will be visually assessed in multiple views by 2D echocardiography
- Non-contrast CT images will be acquired using a 64 slice or dual-source scanner
- Valve leaflet calcification will be measured by the volumetric method that identifies calcium within the valve leaflets
- The total volume of calcification over the 3 valve leaflets will be calculated and expressed in mm3
The RESILIENCE trial will be the first prospective study that uses both clinical and imaging definitions of SVD to provide a comprehensive description of all stages of SVD and determine the long-term (11 years) durability of the RESILIA tissue in a aortic valve.
For a more detailed overview of the RESILIENCE study methodology, please download the clinical summary.
Featured Products
Explore our current RESILIA tissue products
References
- Tod reference is: TJ Tod et al. Cardiovasc Eng Technol 2021;12:418-25.
- Flameng W, et al. J Thorac Cardiovasc Surg. 2015;149:340–5.
- Bartus K et al. Eur J Cardiothorac Surg 2021;59:434-41.
- Bavaria JE et al. Ann Thorac Surg 2022 doi: https://doi.org/10.1016/j.athoracsur.2021.12.058
- Mumtaz MA, Bavaria JE, Griffith B, et al. Presented at the Society of Thoracic Surgeons Annual Meeting, January 2022.
- Bavaria JE, et al. Presented at the American Association for Thoracic Surgery Annual Meeting, May 2022.
- Pibarot P, et al. Structural Heart. 2020;4(1):46-52.