INSPIRIS RESILIA Aortic Valve

Right for today.
Ready for tomorrow.

The right choice today.
Because today, your patients want an improved quality of life and the potential to expand their future options.
Feel confident with a design and features you can trust.
The INSPIRIS RESILIA aortic valve incorporates features of the trusted Edwards PERIMOUNT and Magna Ease aortic valve platforms to enhance ease of implant.
The INSPIRIS RESILIA valve delivers on the promise of better ongoing patient quality of life without the inconvenience of monitoring, dietary restrictions and reduction of participation in active lifestyles typically seen with a mechanical valve.1,2
The INSPIRIS valve possesses many advantages over a mechanical valve:
- Freedom to live a more active lifestyle
- Fewer dietary restrictions
- No need for long-term anticoagulants
- No clicking sound with every heartbeat
Built with RESILIA tissue†, the INSPIRIS valve is designed to offer enhanced tissue anti-calcification technology that will potentially allow the valve to last longer.3

- RESILIA tissue is bovine pericardial tissue treated with a special integrity preservation technology that effectively eliminates free aldehydes, a key factor in tissue calcification, while protecting and preserving tissue.3,4
- RESILIA tissue is the result of a development program of over 10 years, involving more than 100 evaluations of safety and efficacy.
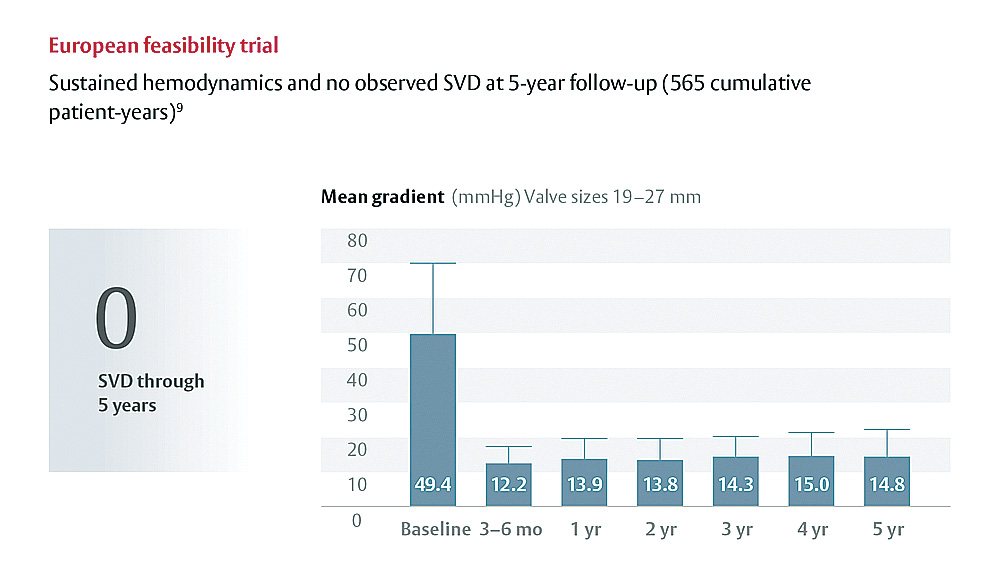
Backed by a strong and growing base of preclinical and clinical evidence supporting its ongoing study of durability and hemodynamic performance.3,5,6
†No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients.


Ready for tomorrow.
Help your patients meet the future confidently, with enhanced options for subsequent valve intervention.
The INSPIRIS RESILIA valve incorporates novel VFit technology, designed to enable valve-in-valve procedures in the future, at a time when patients are older and potentially at a higher risk for complications.
Unlike other valves, the INSPIRIS RESILIA valve is specifically designed to deliver a controlled and predictable expansion during valve-in-valve deployment.*7
*Based on bench data. Refer to device Instructions for Use for important warnings related to VFit technology. These features have not been evaluated in clinical studies to establish the safety and effectiveness of the model 11500A for use in valve-in-valve procedures.
How VFit technology enables controlled expansion
Expanding future possibilities for you and your patients starts today.
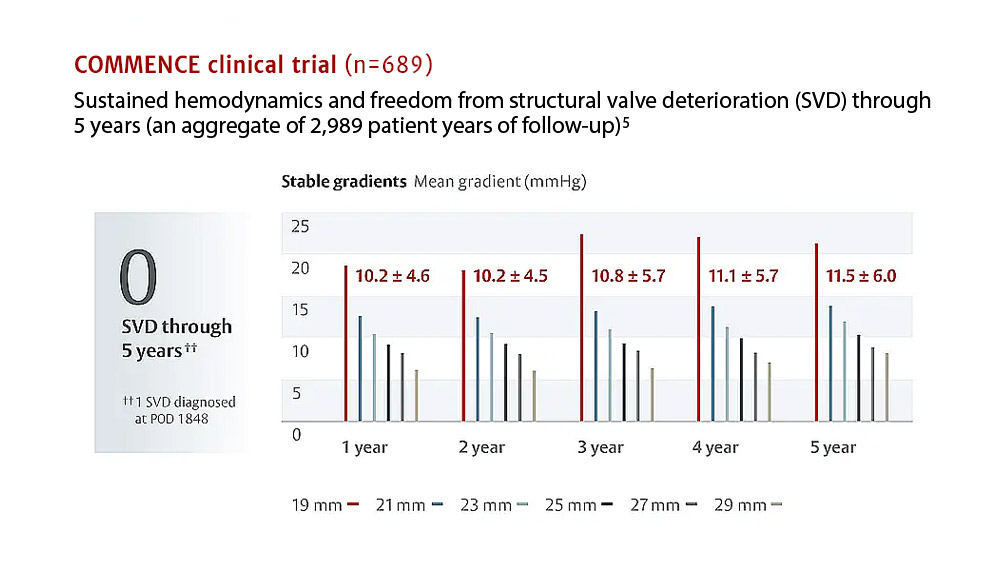
“Patients undergoing a valve replacement are living long lives and remaining more active through their later years, increasing the need for advanced valve replacement technologies. The absence of structural valve deterioration in these patients through five years of follow up is extremely encouraging and highlights the potential of valves containing RESILIA tissue for patients who may otherwise opt for a mechanical valve, which requires long-term use of blood thinning medications.”
John D. Puskas, MD
Principal investigator for the COMMENCE study
VFit technology safety instructions for future intervention
FOR MODEL 11500A SIZES 19–25 MM ONLY.
WARNING: DO NOT PERFORM STAND-ALONE BALLOON AORTIC VALVULOPLASTY PROCEDURES ON THIS VALVE FOR THE SIZES 19 – 25 mm. Although the valve will maintain a stable diameter at implant and during intracardiac conditions, the diameter of this valve will expand if radial force is applied, such as during a balloon aortic valvuloplasty. This may expand the valve causing aortic incompetence, coronary embolism or annular rupture.
The expansion zone is activated by applied radial force. Click video below to view the band expansion.

WARNING: Valve-in-valve sizing in the INSPIRIS valve has only been tested with specific Edwards transcatheter heart valves. Use of other transcatheter valves may result in embolization of transcatheter devices anchored within or result in annular rupture. Refer to device instructions for use for full prescribing and safety information.
*VFit technology has not been observed in clinical studies to establish the safety and effectiveness of the model 11500A for use in valve-in-valve procedures.
The INSPIRIS RESILIA valve is from Edwards Lifesciences, the company trusted by surgeons for more than 60 years to deliver safe, responsible structural heart disease innovation for patients.
References
- Ruel M, et al. Long-term outcomes of valve replacement with modern prostheses in young adults. Eur J Cardiothorac Surg. 2005;27(3):425‐433.
- Kottmaier M, et al. Quality of Life and Anxiety in Younger Patients after Biological versus Mechanical Aortic Valve Replacement. Thorac Cardiovasc Surg. 2017;65(3):198‐205.
- Flameng W, et al. A randomized assessment of an advanced tissue preservation technology in the juvenile sheep model. J Thorac Cardiovasc Surg. 2015; 149:340–5.
- Priev A, et al. Glycerol decreases the volume and compressibility of protein interior. Biochemistry. 1996;35(7):2061‐2066.
- Bavaria JE, Griffith B, Heimansohn DA, et al. Five-year Outcomes of the COMMENCE Trial Investigating Aortic Valve Replacement with RESILIA Tissue. Ann Thorac Surg 2022; doi: https:// doi.org/10.1016/j.athoracsur.2021.12.058.
- Bartus K, Bilewska A, Bochenek M, et al. Five-year Outcomes of Aortic Valve Replacement Using a Bioprosthetic Valve with the Novel RESILIA Tissue: Final Study Results. Presented at Heart Valve Society 2019 Annual Meeting.
- Saxon JT, et al. Bioprosthetic Valve Fracture During Valve-in-valve TAVR: Bench to Bedside. Interv Cardiol. 2018;13(1):20‐26.
- Saxon JT, et al. Complications of Bioprosthetic Valve Fracture as an Adjunct to Valve-in-Valve TAVR, Structural Heart, 2019;3:2, 92-99.