Hemodynamic monitoring for COVID-19 and critically ill patients

Hemodynamic monitoring
Patients who require hospitalization for COVID-19 are at an increased risk of developing conditions such as sepsis, acute lung injury (ALI), and acute respiratory distress syndrome (ARDS).1
Hemodynamic instability is a key contributor to mortality in patients with ARDS, and “successfully managing the complex hemodynamics of the ventilated patient with ARDS is key to patient survival.”2 The majority of patients needing ICU treatment will require mechanical ventilation.3 Existing evidence demonstrates critically ill patients who develop these complications are likely to develop multiple organ dysfunction syndrome (MODS) which significantly decreases the chance for patient survival, increases utilization of limited ICU and hospital resources, and ultimately results in an extended length of stay in both the ICU and hospital.4,5
Four phases of hemodynamic treatment in relation to cumulative fluid balance6
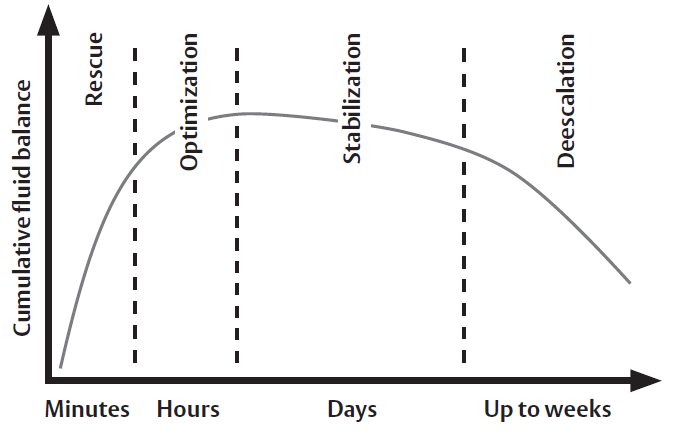
The rapidly changing and complex nature of critically ill patients requires continuous information to help guide the patient through the continuum of care, regardless of the chosen strategy for management. Hemodynamic monitoring can aid clinicians in their treatment of critically ill patients by providing information during the four phases in the time course of critical illness: rescue, optimization, stabilization, and de-escalation. The care modalities and goals of care vary substantially as the patient progresses through the care continuum.6
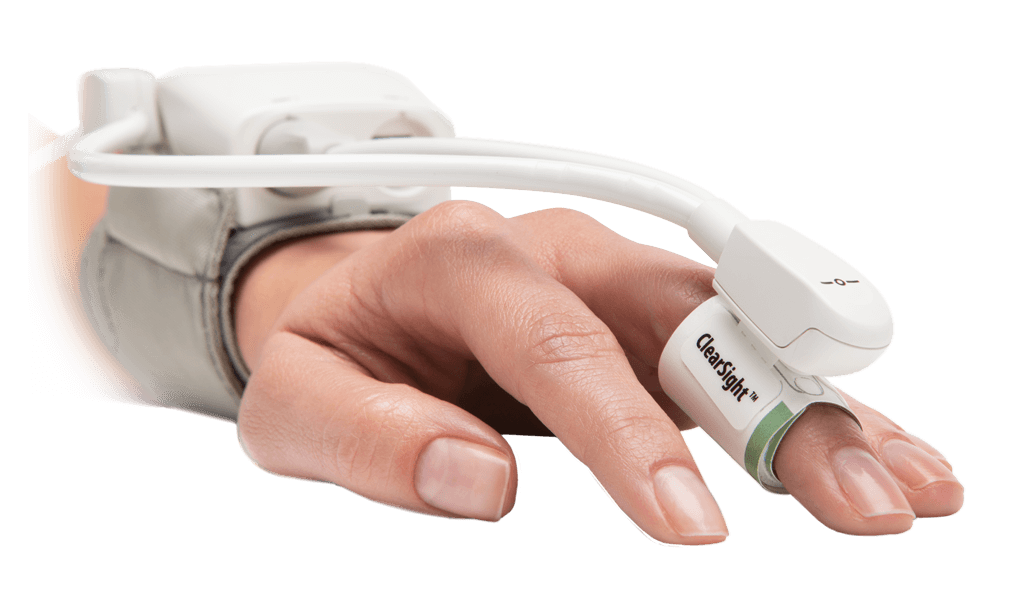
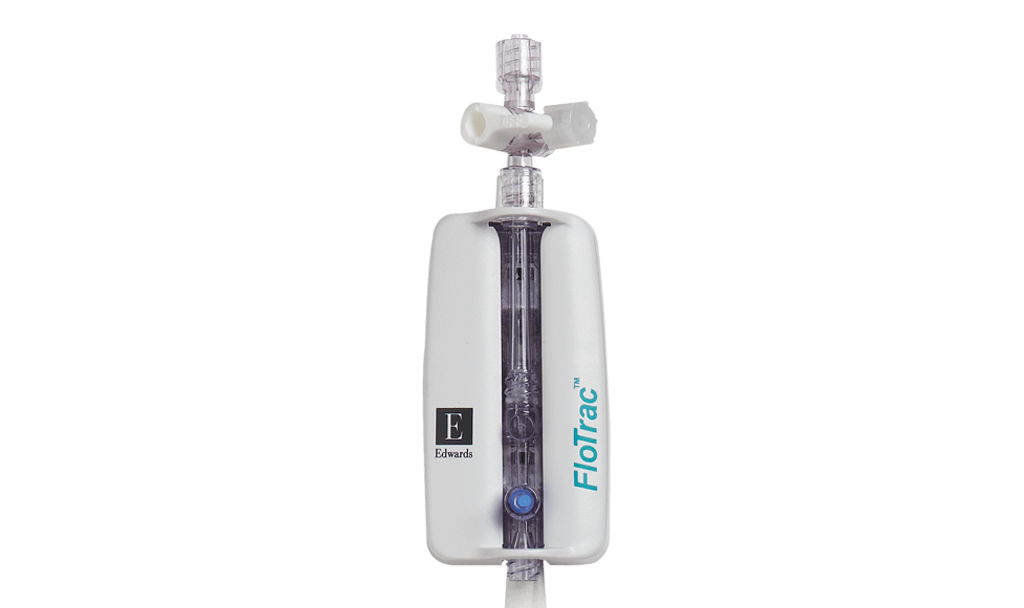
The use of hemodynamic monitoring during the rescue phase includes basic tools to monitor the patient in the initial minutes to hours of care. Continuous noninvasive pressure monitoring (including ClearSight finger cuff) can aid in hypotension management, as well as play a role in the early detection of sepsis, septic shock, and other shock states. Invasive pressure monitoring with disposable pressure transducers (including TruWave pressure transducers) aids in hypotension management and provides access for initial diagnostics, such as blood gases and other labs. In addition to continuous blood pressure, flow-based and dynamic parameters such as cardiac output (CO), stroke volume (SV) and stroke volume variation (SVV) can be obtained (e.g., FloTrac sensor/ ClearSight finger cuff) to give more insight into fluid resuscitation therapy than pressure-based parameters.3,6,7
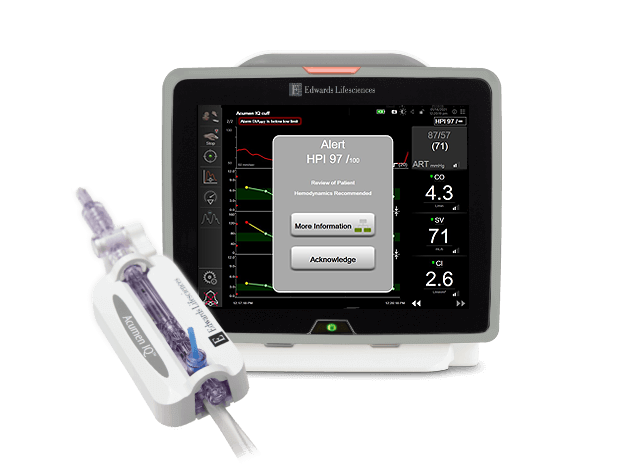
Dynamic parameters can help identify the most appropriate initial therapy, such as helping clinicians decide between volume administration versus initiation of vasopressors. Additionally, advanced hemodynamic parameters provide valuable insight into the adequacy of perfusion, as blood pressure may be preserved despite the presence of inadequate tissue perfusion.7 Newer predictive technologies (Acumen Hypotension Prediction Index software) can now reliably predict the hemodynamic instability which leads to hypotension.8
The optimization phase is time limited (generally up to the initial 24 hours) with the goal to reach optimal perfusion of peripheral tissue and repay any oxygen debt incurred through the course of illness.6 Routine use of arterial lines and central venous catheters are common in patients requiring ventilation and are recommended for therapy requiring vasopressors.7 Vasopressors or inotropes should be started only after an appropriate fluid challenge fails to restore organ perfusion.6
Balance of oxygen delivery and consumption9
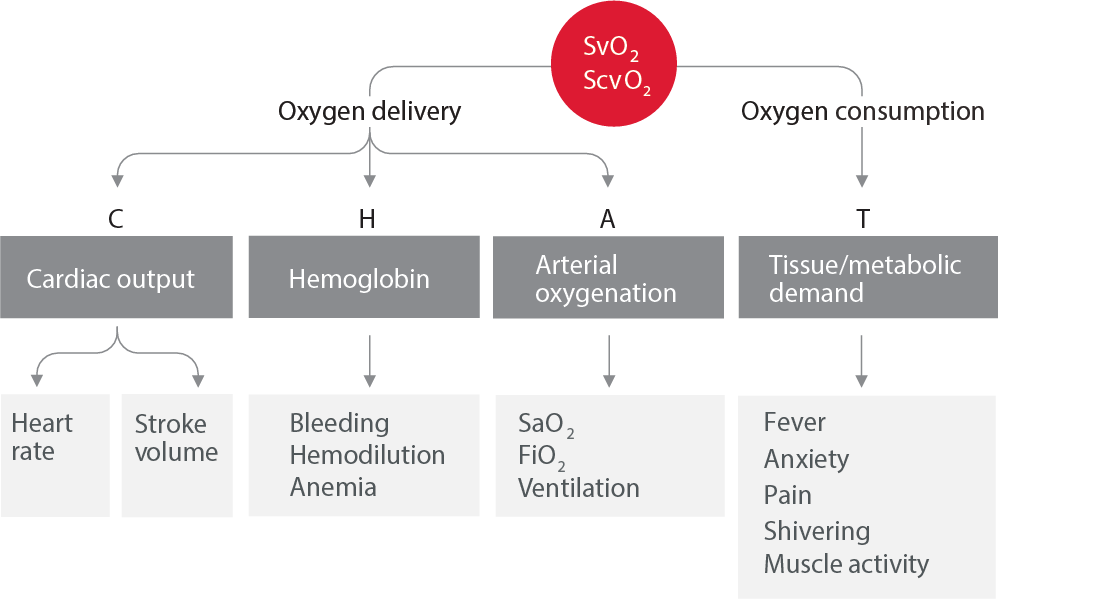
Cardiac output and stroke volume monitoring are recommended in assessing individual patient response to fluids, vasopressors, or inotropes.7 More invasive monitoring, such as the Swan-Ganz pulmonary artery catheter, is indicated in more complex patients such as patients with refractory shock, ARDS, and right ventricular dysfunction.7 These devices provide additional parameters such as right-sided heart pressures, continuous mixed venous oximetry (SvO2), and volumetric parameters such as right ventricular ejection fraction (RVEF) and right ventricular end-diastolic volume (RVEDV).
Hemodynamic monitoring may add additional value in this phase of care, as it allows sequential evaluation of the patient9 and their individual response to therapies.7 This may contribute to early identification of developing complications such as cardiac dysfunction and/or low flow states which may occur.7 For example, patients with ARDS are commonly managed with strategies which may negatively impact patient hemodynamics.1 Restrictive or conservative fluid management may result in inadequate tissue perfusion.10
Fluid imbalance leads to complications10
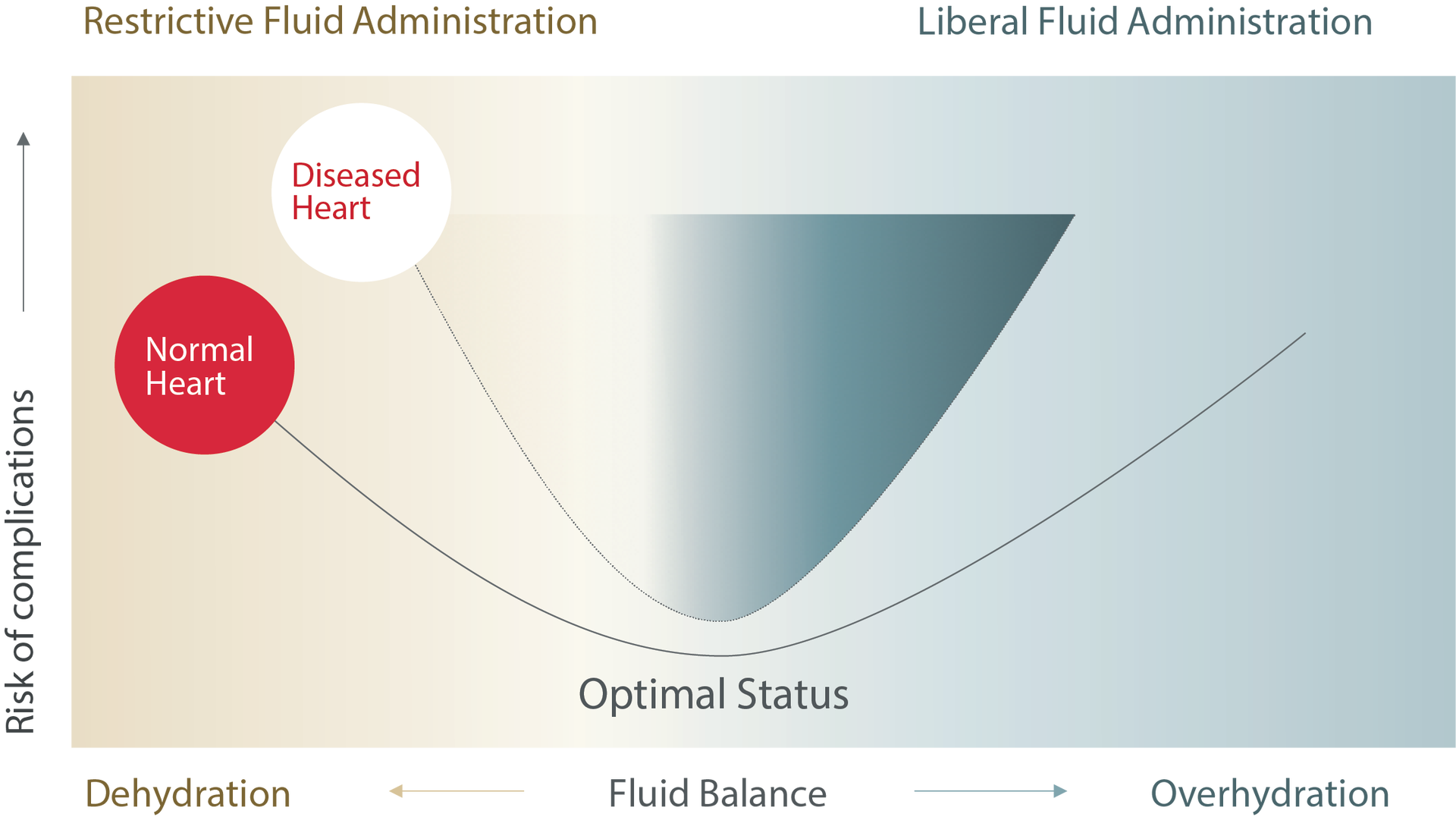
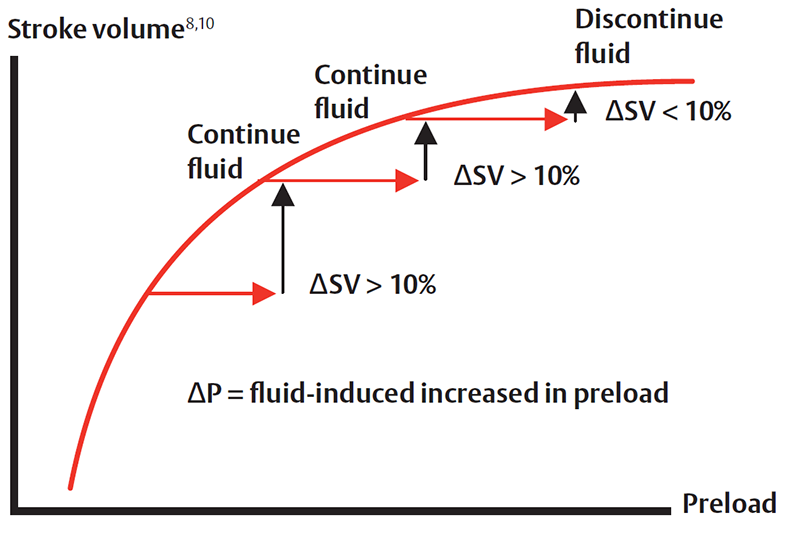
The use of dynamic parameters will allow identification of inadequate tissue perfusion when it occurs and help clinicians verify if fluid administration provides blood flow improvement. In the same regard, these parameters can help limit excess fluid administration when no improvement in blood flow is associated with fluid administration.11
Some therapies used to manage critically ill patients may contribute to inadequate perfusion. For example, the use of high PEEP ventilation is commonly used, and the excessive intrathoracic pressure created may negatively impact right ventricular function.2 Patients requiring high dose vasopressors to maintain adequate MAP may have adversely impacted SV and CO. Also, changes in patient positioning (such as prone therapy) may negatively impact blood flow.2 Other therapies to improve oxygenation, such as inhaled vasodilators, can also significantly alter patient hemodynamics.2
Finally, for the most critically ill patients, tissue oximetry monitoring (such as with ForeSight sensor) may help allow early identification of cerebral desaturations.12,13
As the patient progresses through the stabilization and de-escalation phases, the care shifts from aggressive resuscitation to a state of healing. Hemodynamic monitoring provides continuous data on the patient response during weaning of therapies such as mechanical ventilation, vasoactive medications, and removal of fluid to reduce any positive fluid balance.6 Additionally, hemodynamic monitoring may provide early recognition of developing complications such as sepsis, development of cardiac dysfunction, and hypotension.
In summary, advanced hemodynamic monitoring is highlighted as an essential element in a large variety of guidelines, recommendations, and standards of care for the critically ill patient.1,2,3,6,7
References
- World Health Organization Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: Interim guidance V 1.2
- Viellard-Baron, et al. Experts’ opinion on management of hemodynamics in ARDS patients: focus on the effects of mechanical ventilation. Intensive Care Medicine (2016)
- Alhazzani, et al. Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19). European Society of Intensive Care Medicine and the Society of Critical Care Medicine (2020)
- Vincent & De Backer. Circulatory Shock. New England Journal of Medicine. 2013. 1726-1734.
- Paoli, et al. Epidemiology and Costs of Sepsis in the United States—An Analysis Based on Timing of Diagnosis and Severity Level. Critical Care Medicine Journal. December 2018, Volume 46, Number 12
- Benes et al. Fluid Therapy: Double Edged Sword during Critical Care. BioMed Research International (2015).
- Cecconi et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. (2014)
- Cannesson, et al. Machine-learning Algorithm to Predict Hypotension Based on High-fidelity Arterial Pressure Waveform Analysis. Anesthesiology (2018)
- Nebout, et al. Should We Monitor ScVO2in Critically Ill Patients? Cardiology Research and Practice (2012)
- Bellamy MC. Editorial: Wet, dry or something else? British Journal of Anaesthesia (2006)
- Lopes MR, et al. Goal-directed fluid management based on pulse pressure variation monitoring during high-risk surgery: a pilot randomized controlled trial. Critical Care. (2007)
- Steffen, et al. Using Near-Infrared Spectroscopy to Monitor Lower Extremities in Patients on Venoarterial Extracorporeal Membrane Oxygenation Annals of Thoracic Surgery (2014)
- Wong, et al. Cerebral and Lower Limb near-Infrared Spectroscopy in Adults on Extracorporeal Membrane Oxygenation Department of Surgery Faculty Papers. Paper 76. (2012) http://jdc.jefferson.edu/surgeryfp/76