Freedom from surgical re-intervention at 1 year (n=56)^
Freedom from reintervention with transcatheter pulmonic valve replacement (TPVR) or valve-in-valve at 1 year (n=56)^
Feb 22 is Heart Valve Disease Awareness Day — Learn more about the HVD symptoms at Listen to your Heart
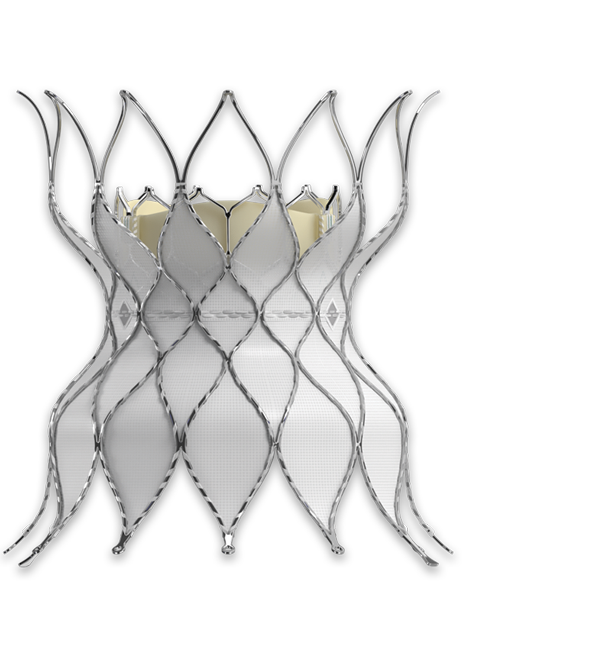

Available for the treatment of pediatric and adult patients with dysfunctional RVOT conduit or surgical bioprosthetic valve in the pulmonic position.
A less invasive alternative to surgery for patients with a history of pulmonary valve intervention.
Freedom from surgical re-intervention at 1 year (n=56)^
Freedom from reintervention with transcatheter pulmonic valve replacement (TPVR) or valve-in-valve at 1 year (n=56)^
The major risks associated with the transcatheter pulmonic valve procedure include death, heart damage potentially requiring surgery, bleeding, blood vessel complications, and irregular heart beat.
All cause death at 1 year† (n=51)^
Endocarditis at 1 year (n=51)^
Valve frame fracture at 1 year (n=51)^
Device success rate (n=53/54)†^
None/trace paravalvular regurgitation at 1 year (n=47)^
^(n=56) COMPASSION S3 trial results using the 20, 23, 26, and 29 mm SAPIEN 3 valve in dysfunctional RVOT conduit or surgical heart valve, valve implant population
† Device success is a composite of
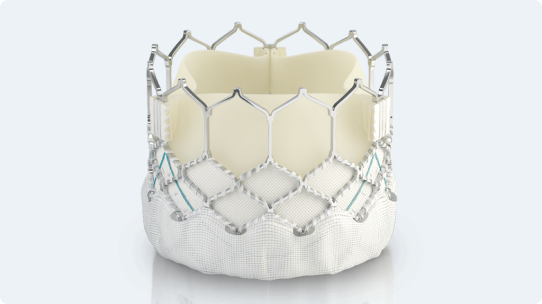
Polyethylene terephthalate (PET) outer skirt is designed to minimize paravalvular leak
Utilizes the same bovine pericardium tissue and processes** as Edwards surgical valves
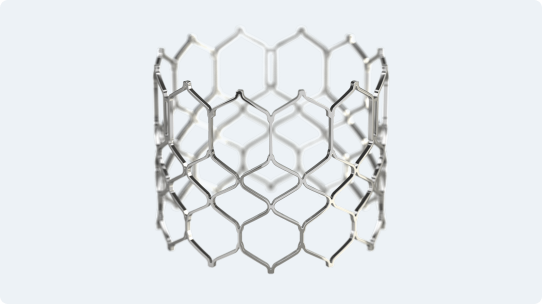
Enhanced frame geometry for low profile delivery
**Excludes RESILIA tissue
Features of the Edwards commander delivery system
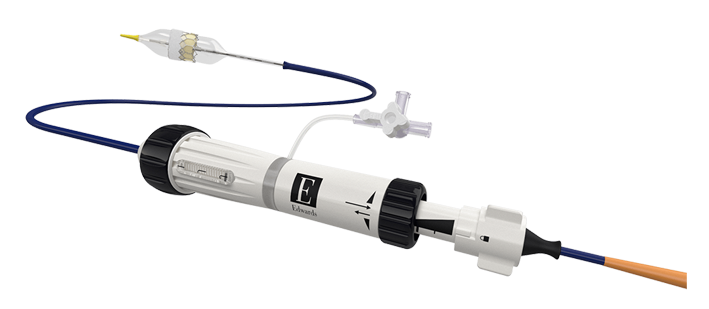

Features of the Edwards eSheath introducer set


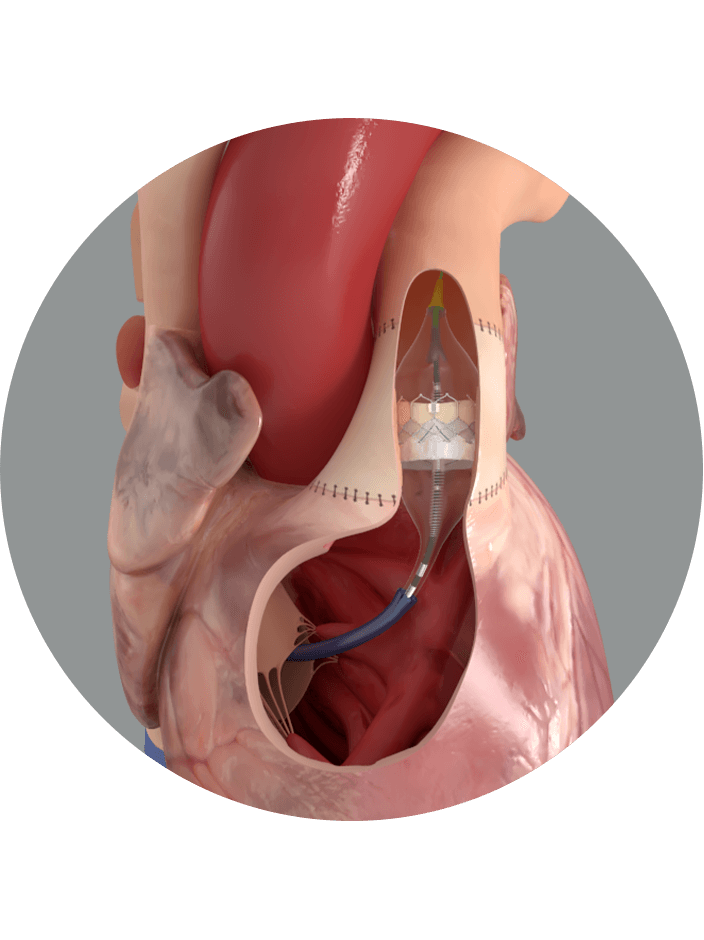
Fewer invasive procedures for more pulmonic patients
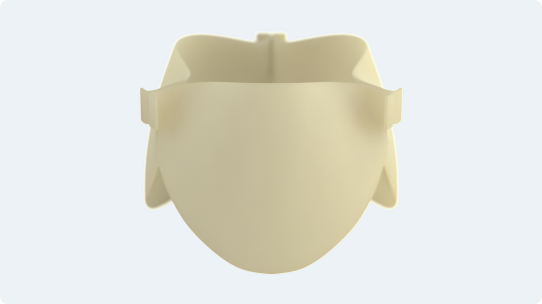
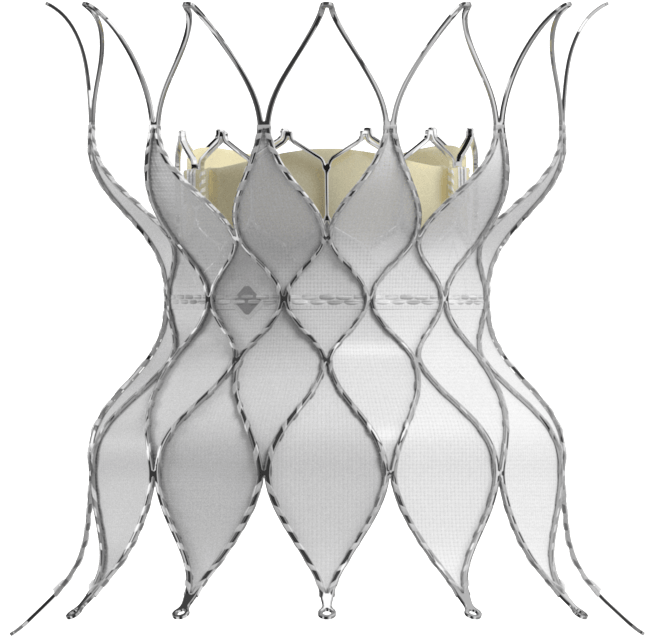
Continue meeting the needs of new patient populations with the Alterra adaptive prestent. It allows you to treat more pulmonic patients with severe pulmonary regurgitation with the SAPIEN 3 valve.
Delivering excellent clinical outcomes with sustained performance1
Single Alterra prestent deployed in desired location (n=60)
Single SAPIEN 3 valve deployed in desired location (n=58/60)
Mortality
Endocarditis
Stent fracture requiring reintervention
Reintervention (n=59)
Mild pulmonary regurgitation (n=53)
Download the Edwards SAPIEN 3 transcatheter pulmonic valve patient brochure.
1. Shahanavaz, Shabana Transcatheter Pulmonary Valve Implantation with the Alterra Adaptive Prestent and SAPIEN 3 Transcatheter Heart Valve: Two-Year Outcomes of the Alterra Pivotal Trial; Presented at PICS-AICS 2023, Washington DC.