
Created for it
Developed with your patient's quality of life in mind

Edwards Lifesciences has developed the MITRIS RESILIA mitral valve to help meet the specialized needs of your patients.

Developed with your patient's quality of life in mind

A saddle-shaped sewing cuff mimics the native mitral annulus

Handles the pressure of the mitral position
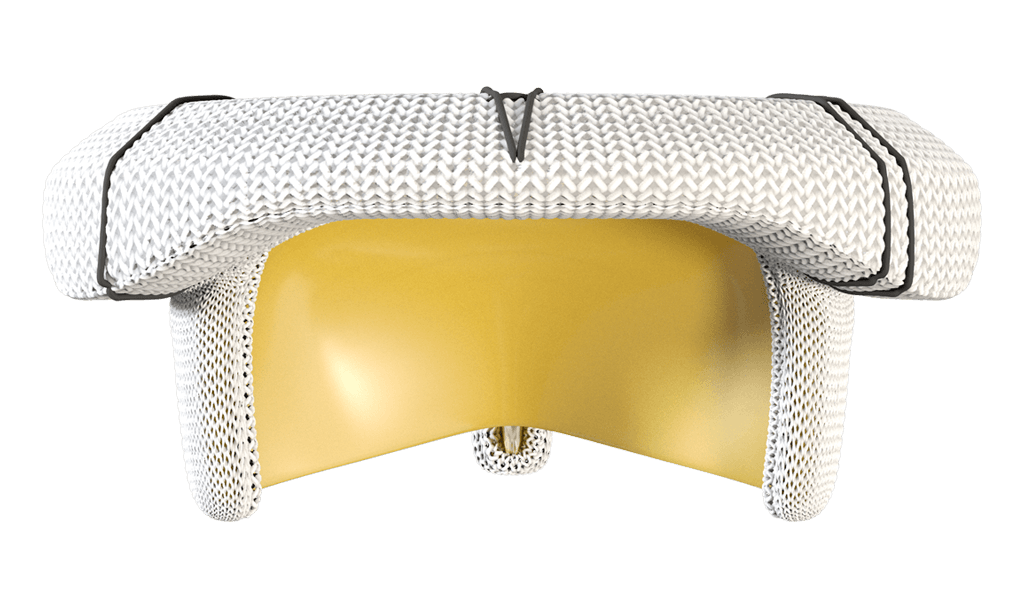
a platform with over 20 years of published clinical durability.1
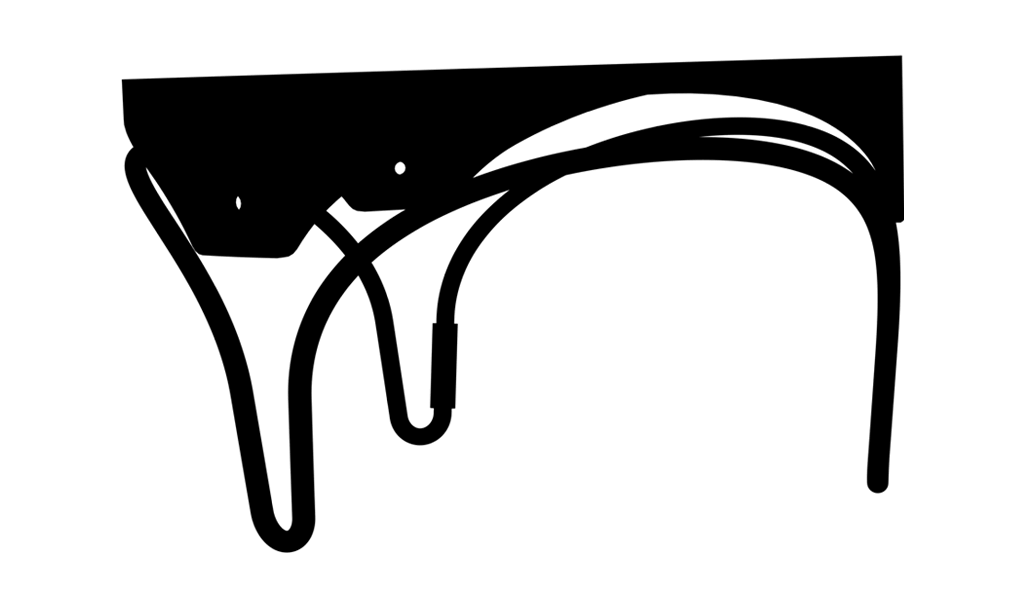
for easy identification of the landing zone for potential future transcatheter interventions.

to eliminate need for rinsing.
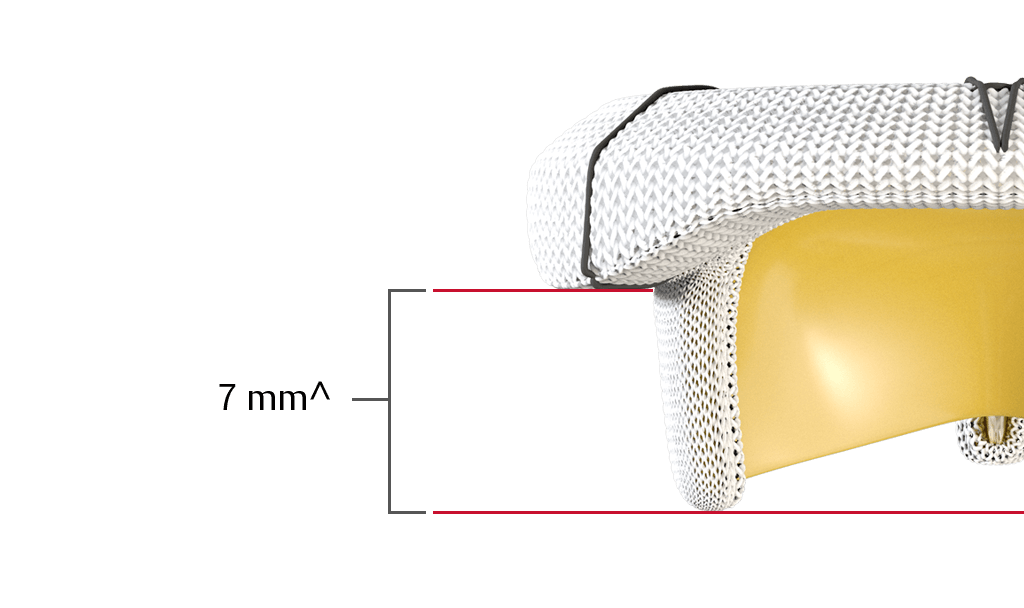
do not obstruct blood flow through the left ventricular outflow tract (LVOT).
^On a label size 25 mm. Anterior Profile Height.
Backed by a strong and growing body of clinical evidence supporting RESILIA tissue’s ongoing study of durability and hemodynamic performance 2, 3, 4

Clinically stable hemodynamics and one incident of structural valve deterioration (SVD) through 4 years in 82 patients.


Clinically stable hemodynamics and zero SVD through 5 years in 689 patients*.
*1 SVD diagnosed at post-operative day 1848


Clinically stable hemodynamics and zero SVD through 5 years in 133 patients.

Built on the trusted Carpentier-Edwards PERIMOUNT valve platform, and made with RESILIA tissue for decreased calcification†, this is the mitral valve developed with your patient's quality of life in mind.
† No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients.

For Indications, contraindications and general warnings related to use of MITRIS RESILIA Mitral Valve, please refer to the detailed Instructions for Use.
The MITRIS valve features RESILIA tissue and is treated with a special integrity preservation technology that effectively eliminates free aldehydes while protecting and preserving tissue.
No clinical data are available that evaluate the long term impact of RESILIA tissue in patients.